Abstract
PRS-080#022 is a 20kD AnticalinTM protein linked to 30kD linear poly-ethylene-glycol that specifically binds to human hepcidin 25, thereby inhibiting its activity. PRS-080#022 is developed for the treatment of functional iron deficient anemia associated with chronic kidney disease or cancer. Elevated levels of hepcidin restrict iron availability and contribute to functional iron deficiency and anemia. Thus, antagonizing hepcidin with PRS-080#022 has the potential to improve iron availability and erythropoiesis, thereby avoiding overload with exogenous iron and reducing the administered levels of Erythropoiesis-Stimulating Agents.
48 healthy male subjects were treated in this placebo controlled, double-blind Phase I study with ascending doses of PRS-080#022 in 6 cohorts at 0.08, 0.4, 1.2, 4.0, 8.0, and 16.0 mg/kg. 6 subjects per cohort received PRS-080#022 and 2 subjects received placebo (NCT02340572). Placebo or active treatments were administered by intravenous infusion over 2 hours. Safety, tolerability, the pharmacokinetics of total and free PRS-080#022, serum hepcidin concentrations as well as parameters of iron metabolism (ferritin, serum iron, transferrin saturation, reticulocytes and hemoglobin) were investigated.
PRS-080#022 was well tolerated. 39 adverse events (AE) were reported during or after treatment in 22 subjects. All such AEs were mild or moderate and no serious AE was observed. Headache was the most frequently observed AE (10 subjects). Otherwise, no association of AEs to specific organs and no apparent dose dependency or difference between placebo and active treatment were observed. Notably, no hypersensitivity or infusion reactions were noted and vital signs, body temperature and ECG were unchanged.
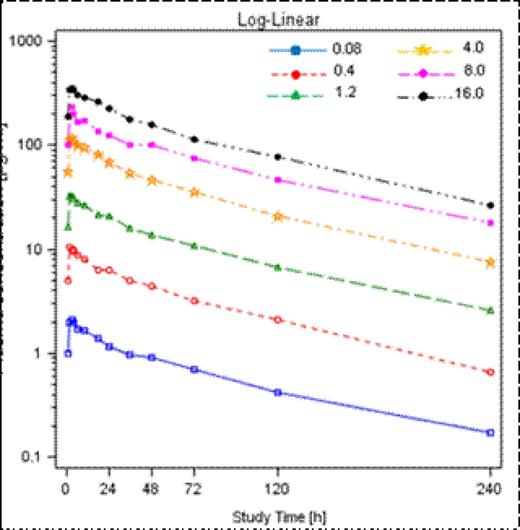
Pharmocokinetics of total PRS-080#022 followed a two-compartment model and was consistent between dose cohorts and within subjects of each cohort (Figure 1). Maximal concentration (Cmax) and area under the time curve (AUC) increased proportionally with dose (Table 1). Cmax was reached about 1 h after the 2 h infusion period (Table 1). The terminal plasma half life (T1/2) of PRS-080#022 ranged from 71 to 81 hours among dose cohorts (Table 1). The volume of distribution was small with 49 to 65 ml/kg, consistent with a distribution mainly to the blood volume.
Administration of PRS-080#022 resulted in a decrease of free hepcidin which was observed already 1 h after start of infusion. PRS-080#022 administration induced a transient increase in serum iron concentration and transferrin saturation (TSAT), with both responses exhibiting a comparable time course and at doses of 0.4 mg/kg and higher. TSAT increased to > 90% in individual subjects. Serum iron concentrations reached about 50 µmol/l in individual subjects and did not further increase with dose. Importantly, the time period at which elevated serum iron concentrations and TSAT were observed increased with dose from about 18 h at 0.4 mg/kg to about 120 h at 16 mg/kg PRS-080#022. This is reflected by an increase of the AUC of the serum iron response relative to baseline and placebo (Table 1). In contrast, ferritin levels were largely unaffected by treatment.
The excellent safety profile and the confirmed activity of PRS-080#022 on iron metabolism observed in healthy subjects warrants further investigations in anemic patients. A study investigating safety, pharmacokinetics and activity on erythropoiesis in anemic end-stage chronic kidney disease patients is in preparation.
aFunded by the European Community FP7 health program grant GA-No. 278408 and supported by the EUROCALIN consortium (www.eurocalin-fp7.eu)
Summary of pharmacokinetic and pharmacodynamic parameters
| PRS-080#022 dose [mg/kg] . | Pharmacokinetic Parameters (group means ± SD) . | Pharmacodynamic Parameter (group means ± SD) . | ||||
|---|---|---|---|---|---|---|
| Cmax [µg/ml] . | AUC0-inf [h*µg/ml] . | Tmax [h] . | T1/2 [h] . | Vss [ml/kg] . | Serum Iron AUC0-240# [h*µmol/l] . | |
| 0.08 | 2.1±0.3 | 162 ± 17 | 2.8 ± 0.4 | 81.2 ± 8.7 | 56.2 ± 8.0 | 39 ± 2807 |
| 0.4 | 10.6 ± 1.6 | 761 ± 163 | 3.3 ± 1.6 | 70.5 ± 27.7 | 54.2 ± 9.8 | 1174 ± 1150 |
| 1.2 | 33.9 ± 4.4 | 2264 ±167 | 2.7 ± 0.8 | 80.0 ± 10.3 | 51.3 ± 4.1 | 958 ± 1178 |
| 4.0 | 120.4 ± 19.6 | 7491 ± 730 | 3.7 ± 3.1 | 73.1 ± 8.9 | 47.8 ± 5.6 | 1579 ± 2222 |
| 8.0 | 246.3 ± 56.8 | 15066 ± 2496 | 4.3 ± 2.8 | 79.6 ± 9.7 | 53.3 ± 9.3 | 1134 ± 2207 |
| 16.0 | 366.2 ± 40.9 | 25572 ± 4075 | 3.0 ± 0.6 | 80.2 ± 11.6 | 64.6 ± 14.6 | 3480 ± 2123 |
| PRS-080#022 dose [mg/kg] . | Pharmacokinetic Parameters (group means ± SD) . | Pharmacodynamic Parameter (group means ± SD) . | ||||
|---|---|---|---|---|---|---|
| Cmax [µg/ml] . | AUC0-inf [h*µg/ml] . | Tmax [h] . | T1/2 [h] . | Vss [ml/kg] . | Serum Iron AUC0-240# [h*µmol/l] . | |
| 0.08 | 2.1±0.3 | 162 ± 17 | 2.8 ± 0.4 | 81.2 ± 8.7 | 56.2 ± 8.0 | 39 ± 2807 |
| 0.4 | 10.6 ± 1.6 | 761 ± 163 | 3.3 ± 1.6 | 70.5 ± 27.7 | 54.2 ± 9.8 | 1174 ± 1150 |
| 1.2 | 33.9 ± 4.4 | 2264 ±167 | 2.7 ± 0.8 | 80.0 ± 10.3 | 51.3 ± 4.1 | 958 ± 1178 |
| 4.0 | 120.4 ± 19.6 | 7491 ± 730 | 3.7 ± 3.1 | 73.1 ± 8.9 | 47.8 ± 5.6 | 1579 ± 2222 |
| 8.0 | 246.3 ± 56.8 | 15066 ± 2496 | 4.3 ± 2.8 | 79.6 ± 9.7 | 53.3 ± 9.3 | 1134 ± 2207 |
| 16.0 | 366.2 ± 40.9 | 25572 ± 4075 | 3.0 ± 0.6 | 80.2 ± 11.6 | 64.6 ± 14.6 | 3480 ± 2123 |
#Response as Area Under the Curve 0-240h over baseline, placebo subtracted
Arithmetic mean plasma concentration time profiles of total PRS-080#022
Moebius:Pieris Pharmaceuticals Inc.: Employment. Feuerer:Pieris Pharmaceuticals Inc.: Other: contracted clinical research. Fenzl:Pieris Pharmaceuticals Inc.: Other: contracted clinical research. van Swelm:PIERIS: Other: member of the EU FP7 Eurocalin consortium. Swinkels:PIERIS: Other: member of EU FP7 Eurocalin consortium. Hohlbaum:Pieris Pharmaceuticals Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal