Abstract
Introduction: Controversy exists regarding the choice of triplet versus doublet salvage therapy among patients with multiple myeloma (MM) experiencing early relapse. Triplet therapies produce deeper responses (CR, ≥VGPR, ORR) and result in prolonged progression free survival (PFS) while doublet therapies demonstrate an improved toxicity profile. We performed a meta-analysis of the RCTs comparing triplet to doublet salvage regimens in early relapsed myeloma patients (1-3 prior lines of therapy). The objective is to test the hypothesis that triplet regimens are tolerable, improve CR, ≥VGPR, ORR rates and would translate to an improved PFS.
Methods: We searched Pubmed, Cochrane databases and ASH, ASCO conference proceedings from 01/2000 through 07/2015 for publications and abstracts to identify the phase III RCTs comparing triplet vs. doublet salvage therapies among patients with relapsed myeloma. A meta-analysis of 4 RCTs (PANORAMA1, MMVAR/IFM 2005-04, ASPIRE, ELOQUENT2 consisting of 2475 patients) was performed using the fixed (Mantel-Haenszel) and random (DerSimonain and Laird) models to calculate the impact of triplets versus doublets (table 1) by evaluating the CR, ≥VGPR, ORR, PFS and toxicities. Mature OS data was not available for the RCTs, hence not included in meta-analysis. The consistency of results (effect sizes) among studies was investigated by means of 2 heterogeneity tests: the χ 2-based Cochran's Q test, and the I2 Statistic. We considered that heterogeneity was present when the P-value of the Cochran's Q test was <.1 and the I2 statistic was > 50%.
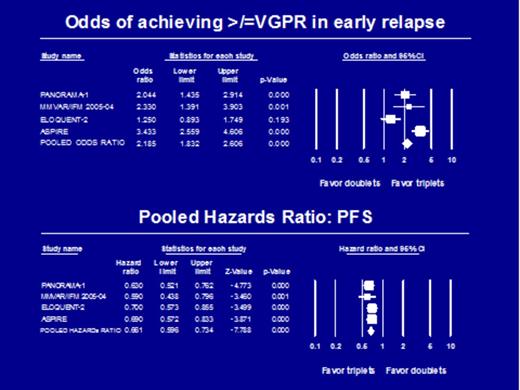
Results: The pooled odds ratios of ORR, ≥VGPR and CR with triplets vs. doublets were 1.935 (P <0.000; 95% CI: 1.614-2.321); 2.185 (P <0.000; 95% CI: 1.832-2.606); 2.461 (P <0.000; 95% CI: 1.888-3.207) respectively, indicating that the odds of achieving higher quality responses are improved with triplet regimens compared to the use of a doublet regimens. The pooled hazard ratio (HR) for PFS was 0.661 (95% CI 0.596-0.734; P =0.000) in favor of triplet regimens (Figure 1). The Q-statistic for PFS (P =0.725; df =3; I2 = 0.00) suggests homogeneity across studies. Though the relative risk of selected ≥grade 3 serious adverse events (G3 SAE) was higher with triplet regimens (diarrhea, fatigue, thrombocytopenia 2.288 (95% CI 1.637-3.197; P =0.000), 1.654 (95% CI 1.263-2.166; P =0.000), 2.434 (95% CI 1.934-3.063; P =0.000), respectively), the overall G3 SAE were comparable with RR 1.498 (95% CI 1.176-1.908; P =0.001) favoring doublets.
Conclusion: Our mixed model meta-analysis demonstrates that triplet regimens in early relapsed myeloma patients result in improved ORR, ≥VGPR, CR and PFS compared to doublets. G3 SAEs are higher with triplet regimens, however this appears to be influenced by the regimen-related toxicity from the PANORAMA1 trial. Appropriate dose modifications or use of selective HDAC inhibitors in future may mitigate the toxicities of the regimen. The pooled estimates ofresponse and survival strongly favor triplets in the early relapsed setting.
Triplet vs. doublet regimens in RCTs
| Trial . | Triplet regimen . | Doublet regimen . |
|---|---|---|
| PANORAMA1 | Panobinostat, bortezomib, dexamethasone | Placebo, bortezomib, dexamethasone |
| MMVAR/IFM 2005-04 | Bortezomib, thalidomide, Dexamethasone | Thalidomide, Dexamethasone |
| ASPIRE | Carfilzomib, lenalidomide, Dexamethasone | Lenalidomide, Dexamethasone |
| ELOQUENT 2 | Elotuzumab, lenalidomide, Dexamethasone | Lenalidomide, Dexamethasone |
| Trial . | Triplet regimen . | Doublet regimen . |
|---|---|---|
| PANORAMA1 | Panobinostat, bortezomib, dexamethasone | Placebo, bortezomib, dexamethasone |
| MMVAR/IFM 2005-04 | Bortezomib, thalidomide, Dexamethasone | Thalidomide, Dexamethasone |
| ASPIRE | Carfilzomib, lenalidomide, Dexamethasone | Lenalidomide, Dexamethasone |
| ELOQUENT 2 | Elotuzumab, lenalidomide, Dexamethasone | Lenalidomide, Dexamethasone |
Nooka:Spectrum Pharmaceuticals: Consultancy; Onyx Pharmaceuticals: Consultancy. Kaufman:Onyx: Consultancy; Celgene: Consultancy; Novartis: Research Funding; Onyx: Research Funding; Merck: Research Funding; Janssen: Consultancy; Spectrum: Consultancy; Novartis: Consultancy. Gleason:Onyx: Consultancy; Novartis: Consultancy; Celgene: Consultancy. Lonial:Janssen: Consultancy, Research Funding; Onyx: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Millennium: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Celgene: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal