Abstract
Background: The outcome for patients with Multiple Myeloma (MM) is highly variable. Understanding the prognosis for a particular patient can help when selecting the intensity of treatment to be used and the frequency of reviews. The International Staging System (ISS) is the current standard used for risk stratification of patients with MM. Recent studies have evaluated the relationship between serum free light chains (sFLC) at diagnosis and the prognosis of these patients. The aim of this study is to evaluate the prognostic value of sFLC ratio (sFLCR) at baseline in newly diagnosed MM in a Southern Spanish population.
Materials and Methods: 180 patients (82 Male:98 Female) with a median age of 69 years (range 61-76) were recruited and followed for up to 7 years. The median follow up of the patients was 35 (18-61) months. sFLC concentrations were measured nephelometrically (Freelite, The Binding Site, Birmingham, UK) and the sFLCR was calculated using the monoclonal sFLC as the numerator. Patients were stratified into two groups according to the median value of sFLCR: group "low" with sFLCR<50 (90 patients) and group "high" with sFLCR≥50 (90 patients). Clinical and laboratory variables (including sFLC, Albumin, Beta-2-microglobulin (B2M), creatinine, hemoglobin, calcium, LDH, M-protein size, plasma cell infiltration and presence of lytic bone lesions) were recorded and evaluated for their impact on patient's outcome. Statistical analysis was performed using SPSS 23. Overall survival (OS) was analyzed by Kaplan-Meier method and significant variables were further analyzed by Cox Regression analysis.
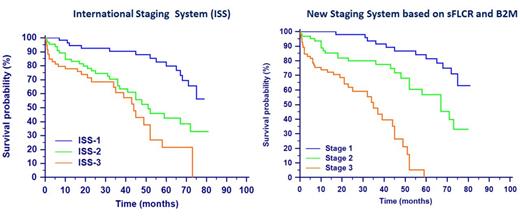
Results: During follow-up there were 72 disease-related deaths: 23 in group "low" and 49 in group "high". The 7-years OS of all patients was 38%, whereas it was 57% and 10% in groups "low" and "high" respectively (HR=5.07 IC95% 3.01-8.55, p<0.0001). Univariate analysis identified age>65 years old, creatinine>2 mg/dL, hemoglobin<10 g/dL, B2M>3.5 mg/L, albumin<3.5 g/dL, involved sFLC≥100 mg/L and ISS stages 2 & 3 as being significantly associated with adverse outcome (Table 1). There was no significant correlation with the others variables. Multivariate Cox regression identified sFLCR (HR=4.65, IC95% 2.71-7.98, p<0.0001) and B2M (HR=2.92, IC95% 1.67-5.11, p<0.0001) as independent risk factors for adverse outcome. The combination of sFLCR≥50 and B2M>3.5 mg/L allows us to include patients in a new model with three risk groups more accurate compared with ISS model (Table 2 and Figure 1).
Conclusions: sFLCR value at diagnosis is an important independent risk factor for poor prognosis in patients with newly diagnosed MM. Our new staging system model based on sFLCR and B2M allow us an accurate risk stratification of patients with MM in our population.
Univariate and multivariate analysis of prognostic factors for patients with newly diagnosed MM.
| Variables . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| HR (95%IC) . | p-value . | HR (95%IC) . | p-value . | |
| Age>65 years | 1.99 (1.17-3.41) | 0.01 | - | |
| sFLC ratio≥50 (involved/uninvolved) | 5.07 (3.01-8.55) | <0.0001 | 4.65 (2.71-7.98) | <0.0001 |
| Involved sFLC≥100 mg/L | 2.19 (1.24-3.90) | 0.007 | - | |
| Total protein (g/dL) | 1.45 (0.91-2.31) | 0.2 | - | |
| Calcium>11 mg/dL | 1.75 (0.87-3.53) | 0.2 | - | |
| Hemoglobin<10 g/dL | 1.95 (1.22-3.12) | 0.005 | - | |
| Creatinine>2 mg/dL | 2.15 (1.25-3.70) | 0.005 | - | |
| Lytic bone lesions | 1.21 (0.74-1.97) | 0.4 | - | |
| B2M>3.5 mg/L | 3.66 (2.16-6.21) | <0.0001 | 2.92 (1.67-5.11) | <0.0001 |
| Albumin<3.5 g/dL | 1.84 (1.14-2.96) | 0.01 | - | |
| M-protein>3 g/dL | 1.86 (1.07-3.21) | 0.03 | - | |
| Plasma cell>10% | 1.57 (0.92-2.71) | 0.1 | - | |
| ISS-2 (vs. ISS-1) | 3.02 (1.56-5.73) | 0.001 | - | |
| ISS-3 (vs. ISS-3) | 4.88 (2.50-9,52) | <0.0001 | - | |
| Variables . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| HR (95%IC) . | p-value . | HR (95%IC) . | p-value . | |
| Age>65 years | 1.99 (1.17-3.41) | 0.01 | - | |
| sFLC ratio≥50 (involved/uninvolved) | 5.07 (3.01-8.55) | <0.0001 | 4.65 (2.71-7.98) | <0.0001 |
| Involved sFLC≥100 mg/L | 2.19 (1.24-3.90) | 0.007 | - | |
| Total protein (g/dL) | 1.45 (0.91-2.31) | 0.2 | - | |
| Calcium>11 mg/dL | 1.75 (0.87-3.53) | 0.2 | - | |
| Hemoglobin<10 g/dL | 1.95 (1.22-3.12) | 0.005 | - | |
| Creatinine>2 mg/dL | 2.15 (1.25-3.70) | 0.005 | - | |
| Lytic bone lesions | 1.21 (0.74-1.97) | 0.4 | - | |
| B2M>3.5 mg/L | 3.66 (2.16-6.21) | <0.0001 | 2.92 (1.67-5.11) | <0.0001 |
| Albumin<3.5 g/dL | 1.84 (1.14-2.96) | 0.01 | - | |
| M-protein>3 g/dL | 1.86 (1.07-3.21) | 0.03 | - | |
| Plasma cell>10% | 1.57 (0.92-2.71) | 0.1 | - | |
| ISS-2 (vs. ISS-1) | 3.02 (1.56-5.73) | 0.001 | - | |
| ISS-3 (vs. ISS-3) | 4.88 (2.50-9,52) | <0.0001 | - | |
Prognostic value of International Staging System (ISS) and New Staging System based on sFLCR and B2M. NR=not reached. (1)p-value=0.09 between ISS-2 and ISS-3. (2)p-value<0.0001 between stages 2 and 3.
| Risk groups . | N . | Number of deceases n (%) . | 7-years OS (%) . | Median survival (months) . | HR (95% CI) . | p-value . |
|---|---|---|---|---|---|---|
| International Staging System (ISS) | ||||||
| Stage 1 | 56 | 14 (25) | 56 | NR | 1.00 | - |
| Stage 2 | 65 | 30 (46) | 33 | 51 | 3.02 (1.59-5.73) | 0.001 |
| Stage 3 | 59 | 28 (47) | 0 | 44 | 4.88 (2.50-9.52 | <0.0001 (1) |
| New Staging System based on sFLCR and B2M | ||||||
| Stage 1 (B2M<3.5 mg/L and sFLCR<50) | 53 | 12 (23) | 63 | NR | 1.00 | - |
| Stage 2 (B2M>3.5 mg/L or sFLCR≥50) | 62 | 23 (37) | 33 | 67 | 3.02 (1.49-6.13) | 0.002 |
| Stage 3 (B2M>3.5 mg/L and sFLCR≥50) | 65 | 37 (57) | 0 | 35 | 11.60 (5.53-24.33) | <0.0001 (2) |
| Risk groups . | N . | Number of deceases n (%) . | 7-years OS (%) . | Median survival (months) . | HR (95% CI) . | p-value . |
|---|---|---|---|---|---|---|
| International Staging System (ISS) | ||||||
| Stage 1 | 56 | 14 (25) | 56 | NR | 1.00 | - |
| Stage 2 | 65 | 30 (46) | 33 | 51 | 3.02 (1.59-5.73) | 0.001 |
| Stage 3 | 59 | 28 (47) | 0 | 44 | 4.88 (2.50-9.52 | <0.0001 (1) |
| New Staging System based on sFLCR and B2M | ||||||
| Stage 1 (B2M<3.5 mg/L and sFLCR<50) | 53 | 12 (23) | 63 | NR | 1.00 | - |
| Stage 2 (B2M>3.5 mg/L or sFLCR≥50) | 62 | 23 (37) | 33 | 67 | 3.02 (1.49-6.13) | 0.002 |
| Stage 3 (B2M>3.5 mg/L and sFLCR≥50) | 65 | 37 (57) | 0 | 35 | 11.60 (5.53-24.33) | <0.0001 (2) |
Harding:The Binding Site Group Ltd: Employment, Membership on an entity's Board of Directors or advisory committees. Campos:The Binding Site Spain: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal