Abstract
Background: Double-hit lymphomas (DHL) are a subset of diffuse large B-cell lymphoma (DLBCL) with concurrent chromosomal rearrangements involving the MYC and BCL2 or BCL6 genes, and are associated with dismal outcomes with standard upfront therapy. Double expressing lymphomas (DEL) are a subset of DLBCL with co-expression of MYC and BCL2 by immunohistochemistry (IHC), and also have a poor prognosis with standard therapy. While DHL status may be associated with inferior outcomes in patients with relapsed or refractory (rel/ref) disease (Cuccuini, Blood, 2012), little is known about the outcome of DHL patients with rel/ref disease who proceed to autologous stem cell transplantation (ASCT), and no study to date has examined the outcome of patients with DEL following ASCT. We evaluated the prognostic impact of DHL and DEL status in patients with rel/ref DLBCL who underwent ASCT at 2 centers.
Methods: We retrospectively studied patients with rel/ref DLBCL, including transformed indolent lymphoma (TIL), who underwent ASCT at Brigham and Women's Hospital/Dana-Farber Cancer Institute (DFCI) and City of Hope (COH) between 1/2000 and 7/2013. The most recent biopsy prior to ASCT was used for testing when possible. IHC for MYC and BCL2 were performed using the Ventana (MYC: DFCI) and Leica BOND III (MYC: COH; BCL2: DFCI, COH) platforms according to standard protocols. For DEL, IHC cutoffs of ≥ 40% MYC-positive and ≥ 50% BCL2-positive cells were used (Johnson, JCO, 2012). Fluorescence in situ hybridization (FISH) for MYC was performed using LSI MYC dual-color break-apart probes (Abbott Molecular, Des Plaines, IL). MYC -rearranged cases had FISH for BCL2 and BCL6 performed using LSI BCL2 and BCL6 dual-color break-apart probes. DHL was defined as > 20% nuclei with break-apart signals for MYC and BCL-2 and/or BCL-6.
Results: 201 patients with available archival tissue and clinical data were included. The median age was 60 (range 30-77) years; 60% were male; 26% had TIL; the median number of prior lines of therapy was 2 (range 2-5); 99% had prior rituximab; 53% had primary refractory disease or early (< 6 mo) relapse; 60% were in CR by PET at ASCT; and conditioning regimens were: 66% CBV v 16% BEAM v 10% rituximab and/or ibritumomab tiuxetan-BEAM v 8% other. Overall, the 4y progression-free survival (PFS) and overall survival (OS) were 44% and 61%, respectively.
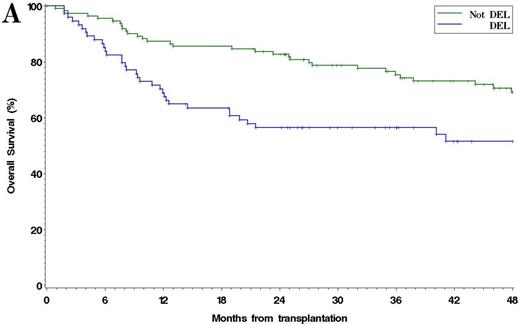
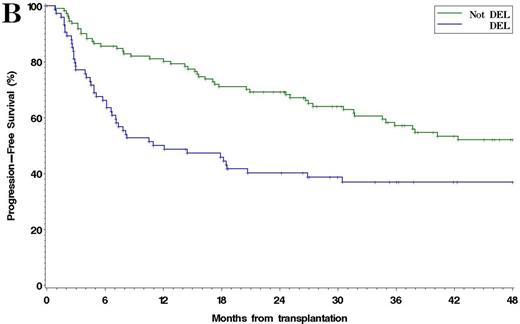
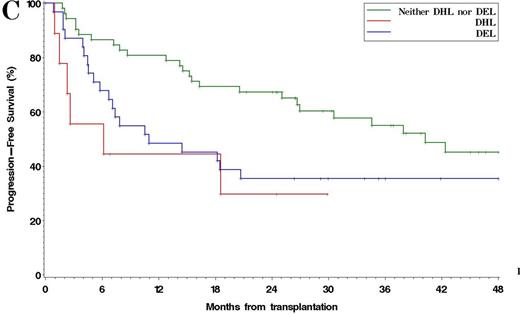
Among 185 patients with complete IHC data, 38% were DEL. The 4y PFS and OS in patients with DEL compared to non-DEL patients were 37% v 52% (p =0.001), and 51% v 69% (p =0.005), respectively [Figure 1]. Results were similar using other reported IHC cutoffs for DEL (e.g. MYC ≥ 40%/BCL2 ≥ 70%, Green, JCO, 2012). Among 93 patients with complete FISH and IHC data available, 13% had MYC rearrangement: 4% were MYC/BCL2 DHL, 3% were MYC/BCL6 DHL, and 2% had rearrangements of all 3 loci. The 4y PFS and OS in DHL v non-DHL were 30% v 42% (p =0.042), and 40% v 57% (p =0.026), respectively. Patients with DEL (excluding DHL) and patients with DHL had similar PFS, which was inferior to non-DEL/non-DHL patients (4y PFS 35% v 30% v 45%, respectively, p =0.026) [Figure 2]. In multivariable models testing pre-ASCT variables, including PET response to salvage, DEL (HR 2.1, p=0.0002), TIL histology (HR 1.8, p=0.009), and SD/PD at ASCT (HR 2.9, p=0.025) were associated with poorer PFS, while DEL (HR 2.0, p=0.004) and SD/PD (HR 3.1, p=0.021) were associated with poorer OS. Neither MYC (≥ 40%) nor BCL2 (≥ 50%) expression alone was independently associated with PFS or OS. When analysis was restricted to the subset of patients with complete IHC and FISH data, DEL (HR 1.9, p=0.023), DHL (HR 2.4, p =0.048), and SD/PD at ASCT (HR 7.6, p =0.009) were associated with inferior PFS. No center effect was observed.
Conclusions: DEL and DHL status are both associated with inferior PFS in patients with rel/ref DLBCL who undergo ASCT, regardless of remission status. Although ASCT remains a potentially curative approach, these patients should be targeted for study of pre- or post-ASCT relapse risk reduction strategies.
(A) Overall survival and (B) Progression-Free Survival after ASCT in DEL vs non-DEL Patients
(A) Overall survival and (B) Progression-Free Survival after ASCT in DEL vs non-DEL Patients
Progression-Free Survival after ASCT in Patients with DEL vs DHL vs non-DEL/DHL
Herrera:Genentech: Research Funding; Pharmacyclics: Research Funding; Sequenta, Inc.: Research Funding. Budde:Atara Biotherapeutics: Consultancy; Seattle Genetics, Inc.: Research Funding; Merck: Research Funding; Ikara Inc: Patents & Royalties. Chen:Seattle Genetics, Inc.: Consultancy, Other: Travel expenses, Research Funding, Speakers Bureau; Genentech: Consultancy, Speakers Bureau; Millennium: Consultancy, Research Funding, Speakers Bureau. Davids:Pharmacyclics: Consultancy; Janssen: Consultancy; Genentech: Other: ad board. Nademanee:Seattle Genetics Inc.: Research Funding; Celgene: Consultancy; Gilead: Consultancy; Spectrum: Research Funding. Siddiqi:Pharmacyclics: Research Funding, Speakers Bureau; Janssen: Speakers Bureau. Forman:Mustang: Research Funding; Amgen: Consultancy. Rodig:Perkin Elmer: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Research Funding. Krishnan:Onyx: Speakers Bureau; Janssen: Consultancy; Millenium: Speakers Bureau; BMS: Consultancy; Jazz: Consultancy; Celgene: Consultancy, Speakers Bureau. Armand:Merck: Consultancy, Research Funding; Infinity: Consultancy, Research Funding; BMS: Research Funding; Sequenta, Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal