Abstract
Introduction: At present, determining OS (Overall Survival) remains the gold standard in clinical trial endpoints which evaluate the role of ASCT in FL. However, in the context of a disease characterized by very long median survivals with a continuous pattern of relapse, and still more with the advent of novel treatments, OS assessments seem elusive. In fact, PFS (Progression Free Survival) is the standard endpoint for new drug approvals in first-line FL, and some other earlier endpoints such 2-year PFS (Casulo et al , JCO 2015) or CR30 (Sargent, 2015) have been recently proposed as potential surrogates and as alternatives to PFS or OS as primary end-points in FL patients treated with 1st line chemoinmunoterapy.
Objective: To assess if 2-year PFS and CR30 are feasible surrogates of OS in the setting of a very long follow-up series of FL patients treated with ASCT.
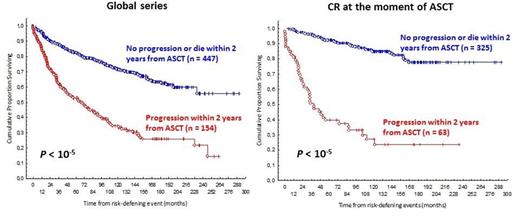
Material and Methods: A total of 626 chemosensitive FL patients (mean age 47 years, male 49%) reported to the Spanish GELTAMO registry and intensified with ASCT between 1989 and 2007 were analyzed. The status of the disease at the moment of ASCT was either in 1st response [203 in 1st CR, 43% of them needing more than one therapy line to reach the CR, and 140 in 1st Partial Response (PR)] or in response after salvage therapy (174 in 2nd CR, 28 in 3rd CR and 81 in 2nd or 3rd PR). In 615 patients the status of the disease was evaluable after ASCT: 569 cases (92%) in CR, 27 cases (4%) in PR and 19 cases (3%) progressed or died. To assess 2-year PFS, two groups were defined: patients with progression of disease (POD) within 2 years from ASCT (early POD) and patients without progression within 2 years from ASCT. Cox model analysis was used to evaluate the association between early POD and OS from a risk - defining event, which is survival from time of POD for early progressors or from 2 years after ASCT for the reference group (Casulo et al, JCO 2015.Appendix). To asses CR30, two groups were defined: patients with and patients without CR at 30 months from ASCT. Cox model analysis was used to evaluate the association between being or not in CR at 30 months from ASCT.
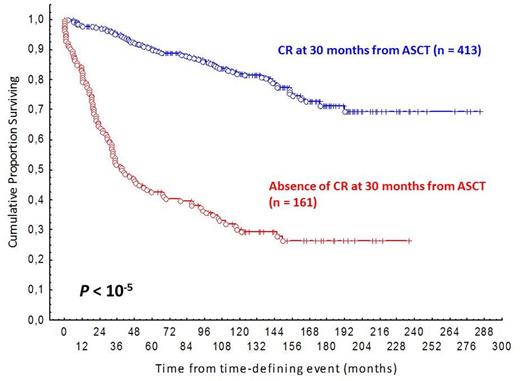
Results: Median follow-up is 12.2 years from ASCT and 14.2 years from diagnosis. Of the assessable patients, 31% were in the high-risk FLIPI group and 40% in the high-risk FLIPI 2 group. 30% of patients received rituximab prior to ASCT. Globally median PFS and median OS are 11 and 21 years, respectively. Patients transplanted in PR (n=221; 35%) had a worse OS than those transplanted in CR (n=405, 65%): HR 2.45 (95% CI, 2.2-2.7; P<10-5) (fig. 1). Similar findings were found in the subgroup of patients transplanted as first line therapy HR 2.59 (95% CI, 2.3-2.8; P<10-5,), or as salvage therapy HR 2.69 (95% CI, 2.2-2.7; P<10-5). Of the global series, 25% (n=154) had an early POD, 71% (n=447) didn't progress or die within 2 years from ASCT and 4% (n=25) died without POD less than 2 years after ASCT, and were excluded from the analysis. Of the 405 patients transplanted in CR, 16% (n=63) had an early POD, 80% (n=325) didn't progress or die within 2 years from ASCT and 4% (n=17) died without POD less than 2 years after ASCT, and were excluded from the analysis. Early POD is associated with reduced OS in all context [the global series: HR 6.8 (95% CI, 6.5-7.1; P=10-5) (fig. 2); the patients transplanted in CR: HR 8.9 (95% CI, 8.4-9.3; P<10-5 (fig. 2); where a "plateau" is found, and the patients transplanted in only PR: HR 3.9(95% CI, 3.4-4.3; P<10-5). In the subgroup of patients treated with rituximab before ASCT (n=179) both, the fact of being transplanted in CR and the absence of an early POD, remain associated with an improve OS: HR 1.82 (CI 95%, 1.5-2-1; P=0.05, and HR 7.5 (CI 95%, 7.2-7.8; P=10-5), respectively. In the same way; for patients responding to ASCT (n=596; 97%), the absence of a CR at 30 months from ASCT (n=161), is associated with a reduced survival: HR 9.8 (95% CI 9.5-10.3; P>10-5), fig. 3.
Conclusion: 2-year PFS and CR30 could be used as subrogates for OS and as primary end points, not only in FL patients treated with 1st line chemoinmunoterapy, but also in FL intensified with an ASCT. To our knowledge this is the first study to establish that early relapse after ASCT is predictive of poor survival in FL patients; in both, patients treated or not with rituximab previously to the ASCT. This finding is even more evident in patients transplanted in CR. Likewise, best response achieved before ASCT is a robust prognostic factor for OS in FL patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal