Abstract
Background. About 70% of chronic myeloid leukemia (CML) patients achieve early molecular response (BCR-ABLIS2 10% at 3-months) that lead to 5-years overall survival close to 95%. However, CML patients remain heterogeneous group and several studies in recent years were aimed to personalize treatment based on individual patients' characteristics. Our group previously put forward a hypothesis about the prognostic value of individual BCR-ABL declinerate in the first three months of CML therapy1,2. The ratio BCR-ABL at 3 months to baseline had chosen as 0.1 as best cut-off value to predict MMR at 12 months. The aims of this study were to validate our prognostic method in larger group of patients and compare these results according to CML prognostic scores.
Patients and methods. Fifty-five patients (median age, 52 years; range 19-84; 24 male and 31 female) with chronic phase CML were included in the study. Patients' distribution for Sokal risk groups were as follows: low-30 / intermediate-15 / high-10. Six patients had EUTOS high-risk. Forty-two patients started treatment with Imatinib 400 mg/day, 12 patients started with Nilotinib 600 mg/day and 1 patient started with Dasatinib 100 mg/day. Median BCR-ABL transcript levels was 41.38% at diagnosis, range 3.39-3185.36% (IS). The ratio of BCR-ABL levels at 3 months to baseline for each patient was calculated. In addition, we calculated ratio of BCR-ABL levels at 3 months to BCR-ABL levels at 1 month for 13 patients. Comparison was made of the predictive sensitivity to achieve early molecular response at 3 months (10% by IS) and according to prognostic CML scores (Sokal and EUTOS). We also assessed positive likelihood ratio (LR) value for the probability of achieving MMR between patients' stratification methods. Statistical analysis was conducted with Fisher exact test and sensitivity-specificity analyses.
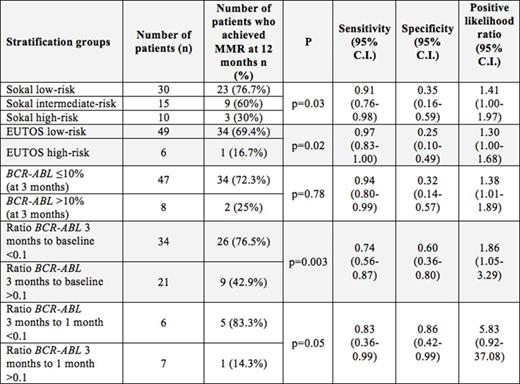
Results. Twenty-six out of 34 patients (76.5%) with ratio of BCR-ABL levels at 3 months to baseline below than 0.1 achieved MMR at 12 months, while only 9 of 21 patients (42.9%) with ratio more than 0.1 had optimal response (LR = 1.86 (1.05 - 3.29); p=0.003). Ratio of BCR-ABL levels at 3 months to 1 month showed much better results with the same (0.1) cut-off value - 5 out of 6 patients (83.3%) with ratio BCR-ABL at 3 months to 1 month below than 0.1, while only 1 patient (14.3%) with ratio more than 0.1 achieved optimal response (LR = 5.83 (0.92 - 37.08); p=0.05), respectively. Application of early molecular response at 3 months (10% by IS) yielded worse discrimination results: 34 of 47 (72.3%) patients with BCR-ABL level ²10% at 3 months, whereas 2 of 8 (25%) patients with BCR-ABL >10% had MMR at 1 year (LR = 1.38 (1.01 - 1.89); p=0.78), respectively. CML prognostic scores results had the following sensitivity-specificity results: for Sokal - low-risk 23 of 30 (76.7%), intermediate-risk 9 of 15 (60%) and 3 of 10 (30%) high-risk patients achieved MMR at 1 year (LR (low+intermediate)/high = 1.41 (1.00 - 1.97); p=0.03); for EUTOS-score - low-risk 34 of 49 (69.4%) and only 1 of 6 (16.7%) high-risk patients had achieved MMR at 12 months (LR = 1.30 (1.00 - 1.68); p=0.02). Furthermore, application of our ratio cut-off value among patients with BCR-ABL level ²10% at 3 months allowed us to revealed additional 6 high-risk patients have not reached MMR at 1 year of therapy (Table 1).
Conclusion. Our study showed that individual rates of BCR-ABL decline from baseline to 3 months and to 1 month had better LR than CML prognostic scores (Sokal, EUTOS) or early molecular response achievement (BCR-ABL levels ²10% at 3 months) and might be useful as an optimized predictors of outcome for CML patients (MMR at 1 year of treatment).
1 Fominykh M., ShuvaevV., Martynkevich I. et al. ELN Frontiers Meeting ÇWhere science meets clinical practiceÈ 16-19 October, 2014, Berlin, Germany. Abstract book: 11.
2 Shuvaev V., Fominykh M., Martynkevich I. et al. Blood (56th ASH Annual Meeting Abstracts), 2014; 124 (21): 5529.
The patient numbers of achieving MMR at 12 months of therapy in various stratification groups with sensitivity-specificity characteristics
The patient numbers of achieving MMR at 12 months of therapy in various stratification groups with sensitivity-specificity characteristics
Chelysheva:Novartis Pharma: Consultancy, Honoraria; Bristol Myers Squibb: Honoraria. Turkina:Bristol Myers Squibb: Consultancy; Pfizer: Consultancy; Novartis Pharma: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal