Abstract
Introduction
Telomeres are specialized DNA structures found at the end of linear chromosomes, which in humans contains the repetitive DNA sequence, (TTAGGG)n and associated proteins. Telomere length (TL) is important for replicative capacity of cells, and, in somatic cells, telomere length shortens with each cell division. Once a critically short length is reached, cells enter senescence or undergo apoptosis. In the general population, TL varies greatly and declines with age. Chemotherapy can increase the rate of telomere shortening, although these findings have not been consistently demonstrated. There is evidence, mostly in adults, suggesting that patients with shorter TL experience increased toxicity from cancer treatment. Patients with the short telomere syndrome, Dyskeratosis Congenita undergoing hematopoietic stem cell transplantation have increased rates and degree of organ toxicity when given myeloablative conditioning. In the pediatric population there have been no studies assessing the relationship between TL and rates of toxicity after chemotherapy, and few investigating telomere dynamics following chemotherapy. We undertook a retrospective analysis to investigate the relationship between TL and chemotherapy toxicity, and also telomere dynamics in children treated for acute lymphoblastic leukemia (ALL).
Methods
Patients enrolled on the Australian and New Zealand Children's Hematology and Oncology Group's (ANZCHOG) Study 8 for ALL at the Children's Hospital at Westmead and the Sydney Children's Hospital from October 2002 to November 2011 who had provided consent and who had stored samples suitable for TL analysis were included in the study. Organ toxicity information was collected from the Study 8 database, as well as examination of patient medical records and pathology information systems. Liver and renal toxicities were documented based on abnormalities in transaminases, bilirubin and creatinine respectively. Pulmonary and neurotoxicities were determined through medical record and imaging findings. Standard common terminology criteria for adverse events (CTCAE) criteria were used to systematically grade toxicity. Infectious disease information and intensive care admissions as well as time to complete each cycle of therapy were used as surrogate markers for toxicity and bone marrow recovery. Survival and relapse rates were also analyzed.
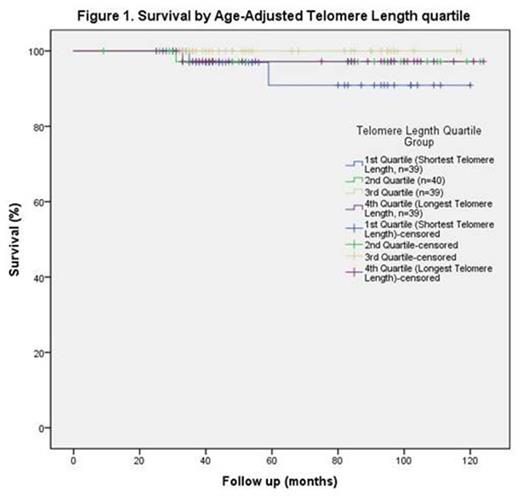
Relative TL was measured using a quantitative PCR technique on DNA extracted from mononuclear cells taken at Day 79 following commencement of induction and consolidation therapy, and also at the end of treatment, typically 24 months from diagnosis. The relative TL was converted to an age adjusted TL (AATL) by subtracting the expected relative TL (i.e. 50th percentile for age of the patient) from the measured relative TL, so that patients of all ages could be analyzed together. For analysis the cohort was separated into four groups based on AATL quartiles.
Results
In all, 460 patients with research consent were enrolled on ANZCHOG ALL Study 8 at the 2 hospitals included in this study. Of these 157 patients with AATL measurement and toxicity information were included in our analysis with 149 being standard or medium risk. The median age of diagnosis was 4.79 years (range 1.1 - 17.89) with a median follow up of 53 months (range 9-124 months).
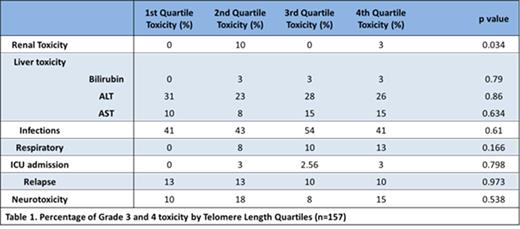
The median AATL on Day 79 was 0.035 (range -0.41 to 0.73). The average change in TL from day 79 to end of treatment was -0.126 (range -0.81 to 0.40), which is equivalent to approximately 8-10 years of natural ageing. There was no significant association between survival (Figure 1) or rates of grade 3 or 4 organ toxicity, relapse (Table 2) or bone marrow recovery and AATL. Renal toxicity was significantly increased in the second shortest quartile, however numbers are small (4 patients in second quartile vs 1 in fourth quartile).
Conclusion
There is an increased rate of telomere attrition during treatment for childhood ALL, however telomere length does not appear to be associated with increased rates of organ toxicity.
Support: NHMRC APP1057746 and NHMRC GNT1056667
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal