Abstract
Background: Adult relapsed/refractory acute lymphoblastic leukemia/lymphoma (rALL) carries a very poor prognosis with limited options for effective salvage therapy. To date, there has been only a single retrospective report on the use of clofarabine in adult rALL demonstrating CR rate of 31% in this setting (Barba P et al. 2012).
Aim: To evaluate the efficacy and toxicity of clofarabine based therapy in a relatively large cohort of adult rALL patients.
Method: After IRB approval, a retrospective single institution study of all patients (pts) diagnosed with rALL treated with a clofarabine based regimen at Mayo Clinic between 2000 - 2014 was performed. Categorical variables were analyzed using Pearson's chi-squared test while continuous variables were analyzed using the Wilcoxon / Kruskal-Wallis test with P < 0.05 considered statistically significant. Survival estimates were calculated using Kaplan-Meier method and means were compared using log ranks.
Results:
Presenting features: We identified a total of 22 pts with a diagnosis of rALL who received clofarabine based therapy. 20 patients were evaluable and 2 were excluded (one received clofarabine as salvage for positive MRD and the other as systemic maintenance with CNS directed therapy for an isolated CNS relapse).
Among evaluable pts, median age at time of Clofarabine based chemotherapy was 39 years (range 19-65) and 50% were males. Thirteen had B-ALL while 7 had T-ALL. Median leukocyte count was 6 x109 (0.2-28.7), hemoglobin 9.8 g/dL (7-12.9), platelet 65 x109 (12-493), peripheral blood blast 4% (0-88) and bone marrow blasts 80% (0-95) while five pts had extramedullary disease. Median number of prior regimens was 2 (1-7).. 8 (40%) pts were post allogeneic transplant
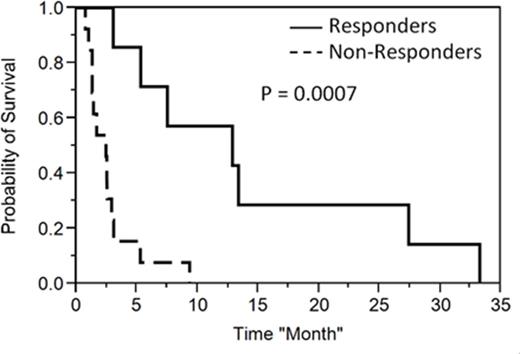
Treatment and Response: 10 pts (50%) received clofarabine monotherapy, 7(35%) clofarabine and cytarabine, and 3 (15%) had clofarabine, etoposide and cyclophosphamide. Overall response rate (CR/CRi + PR) was 35%. Five (25%) achieved CR/CRi, and 2 achieved PR. Among those who achieved CR, 4 out 5 proceeded to further therapy; 2 to allogeneic stem cell transplant and two additional patients received donor lymphocyte infusion (DLI). One pt with PR also proceeded to allogeneic stem cell transplant. In comparative analysis, there were more males 6 (86%) vs 1(14%) in the responding arm (P = 0.014), otherwise the number of regimens prior to clofarabine, type of clofarabine regimen, transplant status, T vs B cell ALL, did not impact response. Median overall survival was 2.4 months (1.3-2.9) in non-responders vs. 12.8 months (3-27.4) in responders (P =0.0007) (Figure 1). All pts who proceeded to transplant or DLI relapsed with a median time to relapse of 6.4 (4.8-13.7) months.
Toxicity: Rash/Hand Foot syndrome was observed in 3 pts (19%), Grade III-IV liver toxicity occurred in 4 (26%) with no permanent sequela. Opportunistic infection occurred in 3 pt (17%).
Conclusion: Clofarabine based chemotherapy was a well-tolerated salvage regimen in heavily pretreated patients and was an effective bridge to definitive therapy in a significant proportion of cases. Prospective evaluation of these findings is warranted.
Al-Kali:Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal