Abstract
Introduction Primary disease relapse remains the leading causeof death inadult acute lymphocytic leukemia (ALL) patients despite the availability of high-dose chemotherapy, targeted drugs and allogeneic hematopoietic stem cell transplantation. Relapsed or refractoryALL especially with high leukemia burden has very poor prognosis. Chimeric antigen receptor-modified T cells (CAR-T) targeting CD19 may overcome many limitations of conventional therapies and induce remission in patients with refractory disease.
Patients and Methods Refractory or relapsed CD19+ ALL adult patients with more than 60% blast cells in the bone marrow at the time of evaluation were enrolled. Eligible patients underwent leukapheresis, and T cells were transduced with a Lenti-virus encoding a CAR construct composed of anti-CD19 scFV linked to 4-1BB and CD3¦Æ signaling domains. To enhance the activity of the transferred CAR-T cells, all patients received lymphodepleting chemotherapy with fludarabine (30mg/m2/day Days -4, -3, -2) and cyclophosphamide (1000mg/m2/day Days -3, -2) followed with 1x10E6 to 10x10E6 CD19CAR-T cells/kg 2 days later. The primary objective of the study was to evaluate the safety and anti-tumor activity of CD19CAR-T cells in ALL. Peripheral blood and bone marrow samples were collected for immunophenotypic, cytokine, and molecular studies at pre-specified times after CD19CART cell infusion.
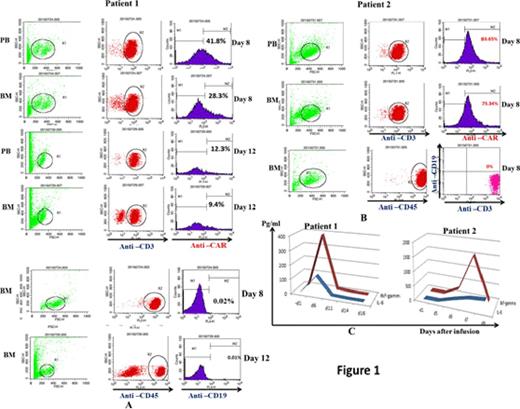
Results 2 patients were enrolled. The patient characteristics are listed in Table 1. All the 2 patients had once achieved complete remission, with MRD<0.01%. Patient 1 suffered from high-risk ALL and achieved CR after 3 courses of induction chemotherapy. Because of lung infection and liver disfunction, he could not receive the 4th chemotherapy. At the time of enrollment on our protocol, he relapsed with 66% blast cells in bone marrow. When the lymphodepleting chemotherapy ended, percentage of his marrow blast cells was 45%. 5x10E6/Kg CAR-T cells were infused. Except for B-cell depletion (Figure 1A, B), the most prominent toxicities included fevers, fatigue and chills (Table 1). The peak blood and bone marrow levels of CAR-T cells were 41.8% and 28.3% of CD3+ lymphocytes in day 8 respectively (Figure 1A). Detailed cytokine analysis showed marked increases of IL-6 and IFN-¦Ã (Figure 1C). Bone marrow blasts were 0.047% and <0.01% in day 8 and day 12 respectively. Patient 2 exhibited persistent chemotherapy refractory disease with 74% blast cells in the bone marrow following salvage chemotherapy. When the lymphodepleting chemotherapy ended, percentage of his marrow blast cells was 77%. 10x10E6/Kg CAR-T cells were infused. This patient suffered severe CRS with marked increases of IL-6 and IFN-¦Ã (Figure 1C). Administered with the IL6-receptor antagonist Tocilizumab she recovered from CRS soon. The peak blood and bone marrow levels of CAR-T cells were 83.65% and 75.34% of CD3+ lymphocytes in day 8 respectively (Figure 1B). Bone marrow blasts were <0.01% on day 8.
Conclusion Our study revealed that CD19CAR-T cells could express robust expansion and anti-leukemia activity in relapsed and refractory ALL patients with high tumor burden. All the 2 patients achieved CR with negative MRD within 12 days after CART cell infusion. No uncontrollable clinical toxicities were manifested. The robust anti-leukemia activity of CD19CAR-T and high-dose CTX chemotherapy might contribute to the good therapeutic efficacy in our clinical trial. More patients will be enrolled in this trial and results will be reported in ASH meeting this year.
Patient characteristics
| Patient . | Age/sex . | Number of prior therapies . | Lymphodepleting chemotherapy . | Marrow blast . | Toxicities . | |
|---|---|---|---|---|---|---|
| Pre-CAR . | Post-CAR . | |||||
| 1 | 51y/M | 3 | Flu(30mg/m2/d -4 to -2) and CTX(1g/m2/d -3, -2) | 66% | <0.01% | Fever Fatigue Chills Anorexia |
| 2 | 29y/F | 22 | Flu(30mg/m2/day Days -4 to -2) and CTX(1g/m2/day Days -3, -2) | 73% | <0.01% | Fever Fatigue Vomit Hypoxemia Hypotension |
| Patient . | Age/sex . | Number of prior therapies . | Lymphodepleting chemotherapy . | Marrow blast . | Toxicities . | |
|---|---|---|---|---|---|---|
| Pre-CAR . | Post-CAR . | |||||
| 1 | 51y/M | 3 | Flu(30mg/m2/d -4 to -2) and CTX(1g/m2/d -3, -2) | 66% | <0.01% | Fever Fatigue Chills Anorexia |
| 2 | 29y/F | 22 | Flu(30mg/m2/day Days -4 to -2) and CTX(1g/m2/day Days -3, -2) | 73% | <0.01% | Fever Fatigue Vomit Hypoxemia Hypotension |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal