Abstract
BACKGROUND AND AIMS
The prognosis of Philadelphia positive (Ph+) acute lymphoblastic leukemia (ALL) patients has improved since the introduction of tyrosine kinase inhibitors (TKI). The inclusion of TKIs in standard ALL protocols allows a great increase in complete molecular responses, but at the price of non negligible toxicities and high rates of toxic deaths. On the other and TKI monotherapy as induction treatment allows to rapidly achieve complete hematologic remission (CR) but only a minority of patients achieve a complete molecular response with high risk of relapse. On the other hand,
In the last years we tested a combination of Fludarabine, Cytarabine, Daunoxome (FLAD) with or without TKIs (mainly Dasatinib) as salvage regimen in relapsed-refractory ALL, with acceptable toxicity and good efficacy. We decided to apply the same schedule in newly diagnosed Ph+ ALL as consolidation treatment after a two months TKI (Dasatinib) monotherapy induction on a minimal residual disease condition.
MATERIALS AND METHODS
FLAD regimen consisted with a three-days administration of Fludarabine 30 mg/sqm followed four hours later by Cytarabine 2000 mg/sqm and Daunoxome 100 mg/sqm. TKI were suspended during chemotherapy administration and were re-administrated starting from day 5. G-CSF was given to all patients from day 4 to complete hematological recovery. FLAD was administrated for up to two cycles; all patients with available donor proceeded to allogeneic bone marrow transplantation (allo-BMT) after FLAD. Minimal residual disease (MRD) was evaluated in all patients after each FLAD either by RQ-PCR for VDJ rearrangements, multicolor flow cytometry (MFC) and RQ-PCR for BCR/Abl.
Ten Ph+ ALL have been treated with FLAD + TKIs from January 2008 to December 2014: six patients received FLAD as salvage regimen, two of them in post allo-BMT setting, whereas four patients were treated frontline, after hematological CR was obtained with Dasatinib + steroids induction. All frontline patients proceeded to allo-BMT after two FLAD. Median age for frontline patients was 50 years (range 29-58), median follow-up was 20 months.
RESULTS
As salvage regimen, 5/6 patients achieved hematological CR after FLAD, with three patients achieving also MFC MRD negativity and clearance of VDJ and BCR/Abl transcript. All patients who did not receive subsequent BMT relapsed, whereas of the two transplanted patients one is still in CR after a follow-up of 38 months.
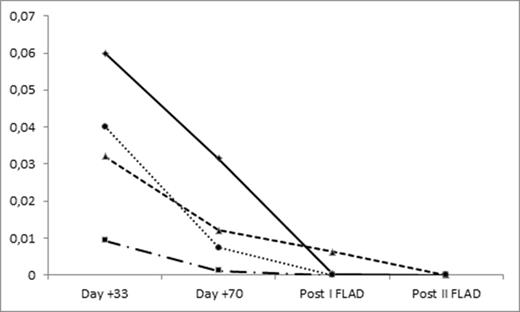
In the frontline setting, all patients received 70 days induction of Dasatinib + Steroids and achieved CR with complete hematological recovery. BCR/Abl transcript could be detected in all patients on BM samples on day 33 and on day 70 (Fig. 1), two patientshad MFC MRD positivity both on day 33 and on day 70, whereas two patients achieved MFC MRD negativity on day 33.
FLAD was very well tolerated, with negligible non hematological toxicity, with a median duration of ANC <500 and PLT <20000 of 7 and 9 days, respectively, slightly higher in the second course. Median time between the beginning of first and second course was 35 days, whereas median time from second course to allo-BM was 44 days.
Two patients achieved BCR/Abl negativity after first FLAD. All patients achieved molecular complete response after the second course (Fig. 1). No patient experienced relapse, whereas one patient died in CR on day +289 after allo-BMT due to myocardial viral infection.
CONCLUSIONS
FLAD has a very good efficacy in adult Ph+ ALL, with an acceptable toxicity profile. Deep responses have been observed in relapsed patients, and all newly diagnosed patients who received FLAD as consolidation regimen had achieved molecular CR before allo-BMT. Achieving complete hematological response with Dasatinib + steroids allowed us to safely administer two FLAD courses.
BCR/abl on bone marrow samples at different timepoints for each of the four patients receiving FLAD as consolidation therapy
BCR/abl on bone marrow samples at different timepoints for each of the four patients receiving FLAD as consolidation therapy
Off Label Use: Use of liposomal daunorubicin in the treatment of ALL.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal