Abstract
Background and purpose: Chemokine (C-C motif) ligand 2(CCL2) is a member of the CC subfamily which displays chemotactic activity for monocytes and basophils. It has played a very important role in many solid tumors and changes in bone marrow microenvironment. However, its role in acute myeloid leukemia (AML) has not yet been clear. In this point, we established a cell line with CCL2 down-expression to explore the effect of CCL2 gene on leukemogenesis.
Methods: Lentivirus with CCL2-knockdown was successfully constructed after screening effective CCL2 shRNA sequence and tranfected into HL-60 cells which was validated on the level of mRNA and protein by real-time PCR and Western blot. The cells coming from parental, sh-Vectors and shCCL2 were detected for cell growth viability by CCK-8 assay, cell cycles and apoptosis by Flow cytometry. We applied exon sequencing technology to identify the gene profiling between the CCL2 knockdown and the control, of which, Cyclin d1 was selected for further experiments as its expression level was significantly downregulated. Then we successfully down regulated cyclin d1 expression in HL-60 by means of RNA interference to detect the cell proliferation through CCK-8 assay, cell cycles and apoptosis through Flow cytometry.
Results: HL-60 cell line expressed the highest level of CCL2 among acute leukemia cell lines (Figure 1). Among 4 pairs of CCL2 interference sequences, only pair 2 had the most efficient potential in knockdown CCL2 expression which was constructed into sh-Vector, GV248, and validated by real-time RT-PCR and Western blot(Figure 2). Low expression of CCL2 significantly decreased HL-60 cell growth. Meanwhile, the CCL2-shRNA-mediated HL-60 cells showed about 12% more cells arrested in G1 phase compared with controls (Table 1, Figure 3). The results of expression profiling showed that there were total 159 genes differentially expressed (Figure 4), of which, ten top pathways were illustrated in Table 2. Cyclin D1 was related to cell cycle, NOD-like receptor signaling pathway, TNF signaling pathway and NF-kappa B signaling pathway which was the lowest expression among cell cycle gene related in HL-60 cells transfect with shCCL2(Table 2, highlighted raw) and further validated by real-time RT-PCR and Western blot (Figure 5). After Cyclin D1 was decreased on the level of mRNA and protein of HL-60, the cell proliferation was evidently slow and cell cycle analysis also indicated a similar pattern of CCL2 (Figure 6).
Conclusion:CCL2 involved in cell proliferation which was mediated by cyclin D1 via blocking more cells at G1 phase.
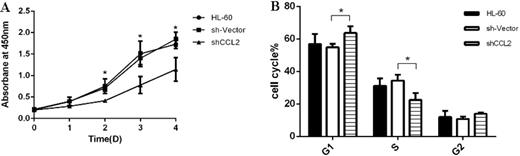
Figure 3. Knockdown of CCL2 inhibits cell proliferation via G1 phase arrested.
A: Down regulation of CCL2 influenced cell proliferation. From day 2 to 5, the proliferation rate of HL-60 cells transfected by shCCL2 grew significantly slower than controls. B: CCL2 played a role in cell cycle process. More cells transfected shCCL2 were arrested in G1 phase compared with controls. *Indicate significant differences with P-values <0.05
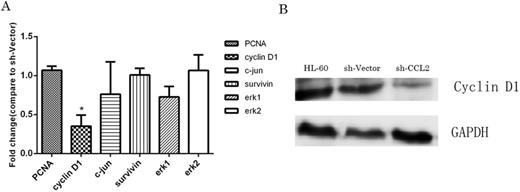
Figure 5. Cylin D1 was the most influenced gene among cell proliferation related genes profile.
A: quantitative RT-PCR analysis showed that mRNA levels of preliferation related genes: PCNA, cyclin D1, c-jun, surviving, erk1, and erk2. Among them, Cyclin D1 was expressed lowest. B: Western blot analysis confirmed that the protein expression of total cyclin D1 was much lower compared with controls.
Figure 6. The effect of Cyclin D1 on cell proliferation.
A: Cyclin D1 was successfully knockdowned in HL-60. mRNA levels were determined by quantitative RT-PCR and revealed that Cyclin D1 expression was knockdowned by the vector of shccnd. B: Knockdown of cyclin D1 inhibits cell proliferation. Compared with control, HL-60 cells with low level of Cyclin D grew significant slowly. C: Down regulation of Cyclin D1 influences more cells arrested in G1 phase compared with control.
*P-values <0.05
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal