Abstract
Acute Promyelocytic Leukemia (APL) is a cytogenetically unique subtype of acute myeloid leukemia (AML), characterized by the presence of the t(15;17)-associated PML-RARA fusion gene. This disease is curable in most patients with all-trans-retinoic acid (ATRA) based therapies, which effectively differentiate malignant promyelocytes. In patients with non-APL AML, most patients with die from their disease and ATRA has little activity. Therefore, research strategies that seek to extend the efficacy of ATRA-based treatment in AML are key avenues of investigation. From our previous studies, an epigenetic analysis of primary AML samples revealed that relative to normal CD33+ cells, loss of RARα2 expression in AML is associated with a reduction in H3K4me2 on the RARA2 promoter (a modification that is associated with transcriptional activation). The mono- and di-methyl lysine demethylase LSD1 (KDM1A) is highly expressed in patients with AML, and its overexpression has been implicated in various other tumors. Based on these data we correctly predicted that the use of small-molecule inhibitors targeting LSD1 (LSD1i) could result in epigenetic reprogramming that enhanced or facilitated the execution of the ATRA-induced differentiation program in AML cells.
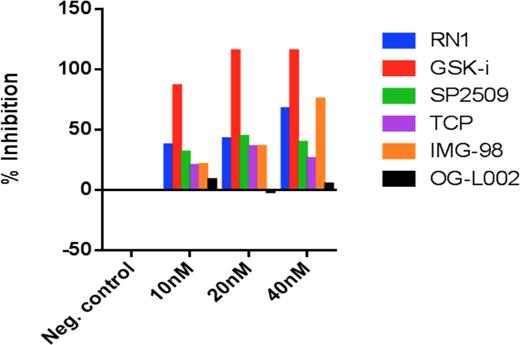
In the current study, we characterized a range of small molecule inhibitors of LSD-1. All the agents tested (RN-1, GSKi, SP2509, TCP, IMG-98 and OG-L002) led to inhibition of LSD-1 in a biochemical assay with varying degrees of potency. From this study, we further characterized the anti-tumor effects of IMG-98 alone and in combination with ATRA. IMG-98 is a novel LSD1 inhibitor relative to drugs of this class with comparatively different specificity, potency, pharmacokinetics, and metabolism. Its greater heavy atom count and chemical complexity contribute to these properties. By fluorine nuclear magnetic resonance (fNMR) and florescent spectrophotometry, the molecule rapidly reacts irreversibly with the FAD co-factor of LSD1 and this polypeptide is necessary to catalyze the reaction. Thermal stability shifts show the inactivated form of the enzyme becomes much more stable suggesting significant structural changes. Treatment with IMG-98 promoted the expression of the cell surface marker CD11b, associated with a differentiated immunophenotype, in both AML cell lines and primary patient material. IMG-98 produced a potent anti-proliferative effect across a range of AML cell lines and also led to growth inhibition of AML blast colony forming ability. In combination studies with ATRA, IMG-98 re-sensitized AML cells to ATRA by reactivating ATRA driven differentiation programs. Post-differentiation apoptosis was more significant for combined therapy (ATRA + IMG-98) than with either agent alone. Heatmap display of unsupervised hierarchical clustering of genes in AML cell lines differentially expressed in response to treatment with combinations of ATRA, IMG-98 or the combination, confirmed that ATRA combined with IMG-98 enhanced the expression of a subset of genes associated with the myeloid differentiation program. Updated studies on mechanisms underpinning mode of action of IMG-98 in this model will be presented.
Taken together, these data demonstrate that ATRA combined with pharmacological inhibition of LSD1, may provide a promising treatment for AML by promoting differentiation and subsequent growth inhibition of AML blasts. A closely related molecule to IMG-98 is currently being optimized in late preclinical development, and clinical trials with this compound are anticipated to start in 2016.
Comparative screening assay for LSD1 inhibition with commercially available agents (LSD1 Inhibitor Screening Assay, Cayman Chemical, Cat# 700120)
Comparative screening assay for LSD1 inhibition with commercially available agents (LSD1 Inhibitor Screening Assay, Cayman Chemical, Cat# 700120)
Rienhoff:Imago: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal