Abstract
Introduction: Minimal residual disease (MRD) monitoring after achievement of hematologic complete remission (CR) in patients (pts) with acute myeloid leukemia (AML) aids in identifying pts at risk of relapse. Evaluating MRD markers for clinical practice is crucial for improving outcomes in AML.
Since mutations in the gene encoding Nucleophosmin (NPM1 mut) occur early in leukemogenesis & are relatively stable during disease course, they represent suitable markers for MRD monitoring. Approximately 30% of AML pts harbor NPM1 mut of which approximately 80% are type A. Some studies already demonstrated the feasibility to use NPM1 mut as MRD markers in AML pts and showed NPM1 mut MRD positivity at different time-points during disease course to be predictive for AML relapse.
Hematopoietic stem cell transplantation (HCT) is a consolidating therapy option offering potential cure to AML pts. Here, we tested the feasibility to use NPM1 mut type A as MRD marker in pts receiving HCT applying digital droplet polymerase chain reaction (ddPCR) methodology. This novel technique offers high sensitivity, specificity & absolute quantification without the need of standard curves, promising inter-laboratory comparability. At the same time cost effectiveness may be superior to standard qualitative real-time PCR making ddPCR a promising new application.
Methods: We analyzed 134 pts (median age 64 years [y], range 38-76y) who received HCT after non-myeloablative conditioning (NMA, 3x30mg/m2 Fludarabine on days -4 to -1 & 2Gy total body irradiation) in CR in our institution between 2000 & 2013. Donors were human leukocyte antigen (HLA)-matched related (n=23; 17.1%) or HLA-matched (n=79; 63.7%) or mismatched (≥1 antigen; n=32; 23.9%) unrelated. Median follow-up was 4.3y for pts alive.
NPM1 mut status was assessed in diagnostic bone marrow (BM) samples by Sanger sequencing of the mutation hot spot in exon 12. All pts with NPM1 mut type A & available BM samples at the time of NMA-HCT (within 28 days before HCT) were analyzed by ddPCR. cDNA was applied to a duplex assay measuring NPM1 wild type (wt) & mutation type A simultaneously using fluorescent labeled probes. The mutation burden, defined as % mut type A copies/wt copies ratio, was normalized to ABL. Samples with NPM1 mut type A burden >0.01% were defined as MRD positive (MRD+) & samples with mutation burden ≤0.01% or <3 positive droplets were defined as negative according to manufacturer's recommendations.
Results: We identified 42 AML pts (24.8%) with NPM1 mut of whom 24 pts had a type A mutation & BM samples at the time of NMA-HCT available. In this set 16 pts (66.7%) were classified to have favorable risk according to European LeukemiaNet (ELN) classification, 7 pts intermediate-I (29.2%) & 1 patient intermediate-II (4.1%) risk. 8 of the 16 pts harbored a FLT3 -ITD.
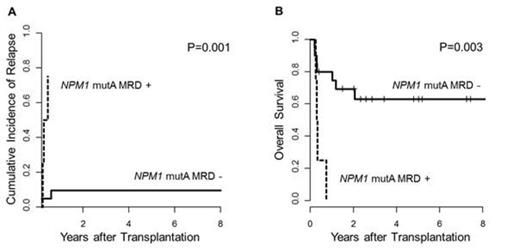
In 4 pts NPM1 mut type A MRD was detectable at the time of NMA-HCT of which 3 pts experienced relapse. The fourth patient died 3 months after NMA-HCT due to transplantation-related complications & no further MRD time-points were available for analysis. In our cohort all clinical relapses occurred within 6 months after HCT. Two pts relapsed without detectable NPM1 mut type A at NMA-HCT. For these two pts there were no follow-up samples for further MRD monitoring after NMA-HCT available. MRD+ status at the time of NMA-HCT associated with a significantly lower cumulative incidence of relapse (P=0.001, Figure 1A) & shorter overall survival (P=0.003, Figure 1B).
Conclusion: Our data demonstrate that ddPCR is a feasible method to determine the NPM1 mut type A burden by absolute quantification. Additionally, we showed the applicability of NPM1 mut type A as MRD marker in AML pts at the time of NMA-HCT. 3 out of 5 pts with clinical relapse were MRD+ at NMA-HCT. Assessing the NPM1 mut type A MRD status at the time of NMA-HCT & possibly at later time-points after HCT by ddPCR might help to identify pts at high risk of relapse. Our results strengthen the observation to use NPM1 mut for MRD monitoring. Consequently, we aim at expanding the applied ddPCR methodology to other NPM1 mut types & testing the validity in the context of clinical studies at various time points before & after NMA-HCT.
Franke:Novartis: Other: Travel Costs; MSD: Other: Travel Costs; BMS: Honoraria. Niederwieser:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal