Abstract
Allogeneic hematopoietic stem cell transplantation (allo-HCT) is an effective, even curative, treatment for patients with high-risk hematologic malignancies. Transplantation from haploidentical donors (haplo-HCT) has been applied for the treatment of hematologic malignancies within the past 2 decades. Bone marrow (BM), G-CSF-primed peripheral blood stem cells (PBSCs), G-CSF-primed BM (G-BM) or the combination of PBSCs and G-BM can serve as stem cell sources for allo-HCT. The optimal source of stem cells in cases of haplo-HCT without ex vivo TCD under myeloablative conditioning is not yet clear. Therefore, we initiated a study of unmanipulated haplo-HCT from PBSCs (haplo-PBSCT) for the treatment of high-risk hematologic malignancies. In this report, we analyzed 89 adult patients who received consecutive haplo-PBSCT to evaluate the efficacy and safety of this transplantation procedure.
PATIENTS AND METHODS
Eighty-nine patients received consecutive haploidentical allo-PBSCT between July, 2007 ¨C June, 2014 at the Chinese PLA General Hospital, Beijing, China (Table 1). PBSCs were freshly isolated and infused into the recipients. The conditioning regimen consisted of Bu (3.2 mg.kg-1.d-1 intravenously, days -10 to -8), Carmustine, 250 mg.m-2, day -5), cytarabine (4 g.m-2.d-1, days -7 to -6), cyclophosphamide (60mg kg-1.d-1, days -4 to -3), and ATG (Thymoglobuline, rabbit; 2.5 mg.kg-1.d-1, days -5 to -2). All transplant recipients received CsA, mycophenolate mofetil, and short-term methotrexate for GVHD prophylaxis.
"High-risk'' hematologic malignancies were defined as: 1) AL with the [t(9;22)(q34;q11)], Flt3-ITD mutation, mixed lineage leukemia genes and complex cytogenetics regardless of disease stage; 2) AML-CR1 after 3 or more cycles of induction, ALL-CR1 after 4 weeks of induction or AL-CR1 with positive MRD after 2 cycles of consolidation; 3) AL beyond CR2 or in non-remission (NR) regardless of cytogenetics, or CML beyond CP1; and 4) T cell lymphoblastic lymphoma in CR and T cell lymphoma resistant to chemotherapy or autologous transplantation. The endpoint of the last follow-up for all surviving patients was January 31, 2015.
RESULTS
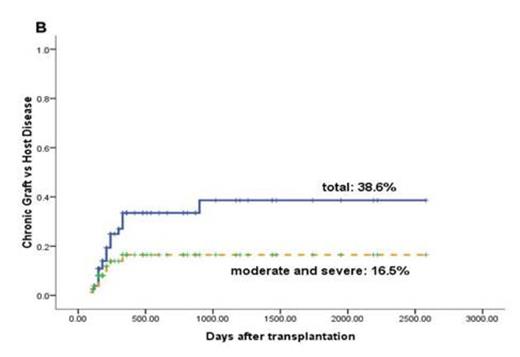
Sustained myeloid engraftment with full donor chimerism was achieved in 89 patients (100%) at a median of 16 (10 - 26) days. Eighty patients (89.9%) achieved platelet recovery in a median of 28 (10 - 207) days. The occurrence of GVHD was showed in Fig 1.
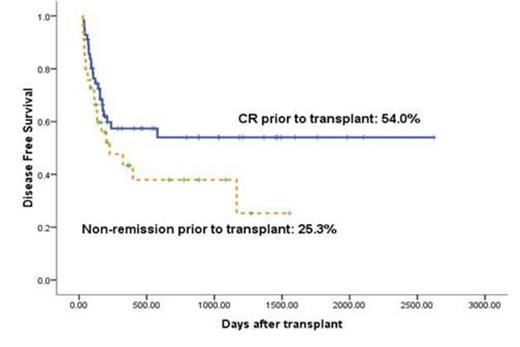
The 3-year of cumulative incidence of transplant-related mortality was 23.4% ± 5.4%. Non-remission status prior to transplant was found to be significantly correlated with relapse (P = 0.006, odds ratio [OR] = 3.17), leukemia-free survival (P = 0.013, OR = 2.48) (Fig. 2) and overall survival (P = 0.03, OR = 2.27).
CONCLUSION
The results described rapid and complete neutrophil engraftment, a low incidence of grade 3-4 GVHD and promising survival in patients with high-risk hematologic malignancies. It demonstrated the reliability of G-CSF-primed PBSCs as a graft source in unmanipulated haplo-HCT under myeloablative conditioning.
Patient and donor characteristics
| . | Cases . | % . |
|---|---|---|
| Gender, n (%) | ||
| Male | 69 | 77.5 |
| Age, y, median(range) | ||
| Patient | ||
| <46 y, n (%) | 28(6-59) | |
| Donor | ||
| >40 y, n (%) | 38(9-61) | |
| Hematologic malignancy, n (%) | ||
| AML | 51 | 57.3 |
| CR1 CR2* | 23 3 | |
| NR*/beyond CR2 | 23/1 | |
| ALL | 20 | 22.5 |
| CR1 CR2 | 10 7 | |
| NR | 3 | |
| CML | 5 | 5.6 |
| CP1* | 2 | |
| AP/CP2 | 1/2 | |
| Lymphoma | 13 | 14.6 |
| CR | 5 | |
| Resistant | 8 | |
| Donor/recipient relationship, n (%) | ||
| Parent | 47 | 52.8 |
| Sibling | 26 | 29.2 |
| Child | 12 | 13.5 |
| Lateral relative | 4 | 4.5 |
| No. of HLA antigens (A/B/DR) mismatched, n(%) | ||
| 1 | 18 | 20.2 |
| 2 | 25 | 28.1 |
| 3 | 46 | 51.7 |
| Second HCT | 9 | 10.1 |
| Graft: | ||
| MNC (108/kg) | 11.04 (5.64-36.46) | |
| CD34+ (106/kg) | 5.83 (2-23.73) |
| . | Cases . | % . |
|---|---|---|
| Gender, n (%) | ||
| Male | 69 | 77.5 |
| Age, y, median(range) | ||
| Patient | ||
| <46 y, n (%) | 28(6-59) | |
| Donor | ||
| >40 y, n (%) | 38(9-61) | |
| Hematologic malignancy, n (%) | ||
| AML | 51 | 57.3 |
| CR1 CR2* | 23 3 | |
| NR*/beyond CR2 | 23/1 | |
| ALL | 20 | 22.5 |
| CR1 CR2 | 10 7 | |
| NR | 3 | |
| CML | 5 | 5.6 |
| CP1* | 2 | |
| AP/CP2 | 1/2 | |
| Lymphoma | 13 | 14.6 |
| CR | 5 | |
| Resistant | 8 | |
| Donor/recipient relationship, n (%) | ||
| Parent | 47 | 52.8 |
| Sibling | 26 | 29.2 |
| Child | 12 | 13.5 |
| Lateral relative | 4 | 4.5 |
| No. of HLA antigens (A/B/DR) mismatched, n(%) | ||
| 1 | 18 | 20.2 |
| 2 | 25 | 28.1 |
| 3 | 46 | 51.7 |
| Second HCT | 9 | 10.1 |
| Graft: | ||
| MNC (108/kg) | 11.04 (5.64-36.46) | |
| CD34+ (106/kg) | 5.83 (2-23.73) |
Acute and chronic GVHD. (A) CI (Cumulative Incidence) of grade 2-4 (continuous line) and grade 3-4 (dotted line) acute GVHD. (B) CI of total (continuous line) and moderate to severe (dotted line) chronic GVHD.
Acute and chronic GVHD. (A) CI (Cumulative Incidence) of grade 2-4 (continuous line) and grade 3-4 (dotted line) acute GVHD. (B) CI of total (continuous line) and moderate to severe (dotted line) chronic GVHD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal