Abstract
Background: HDT-ASCT with TBC conditioning has emerged as a common consolidation strategy for patients (pts) with relapsed/refractory (rel/ref) primary (PCNSL) or secondary (SCNSL) (Welch et al, Leuk & Lymph 2014). In a prospective study, chemosensitive PCNSL pts in first remission after induction with R-MPV (rituximab, MTX, procarbazine and vincristine) proceeding to HDT-ASCT with TBC conditioning, experienced an encouraging 2-year PFS and OS of 75% and 81%, respectively (Omuro et al, Blood, 2015). Three of these patients experienced transplant-related mortality (TRM, 11.5%), which appears greater than HDT-ASCT for other lymphomas. The purpose of this report is to correlate characteristic toxicities of TBC conditioning for CNSL to pre-HDT-ASCT clinical variables.
Methods: The MSKCC IRB approved this retrospective chart review. Eligible pts (n=34) were ≥ 18 years of age with PCNSL or SCNSL that was chemosensitive to induction therapy after which they proceeded to HDT-ASCT conditioned with TBC between December 2006 and April 2015. All pts included were treated outside of prospective clinical trials. Clinically significant grade 3-5 non-hematologic toxicities per CTCAE 4.0 occurring in >20% of pts were recorded from the initiation of conditioning until 6 months post ASCT (Figure 1). Pre-HDT-ASCT variables for analysis include: age, gender, disease (PCNSL or SCNSL), Karnofsky performance status (KPS), hematopoietic cell transplant comorbidity index (HCT-CI), number of prior regimens, prior use of whole-brain radiotherapy (WBRT), and disease status prior to HDT-ASCT (CR/CRu or PR). We evaluated the association of these pre-HDT-ASCT characteristics with the number of clinically significant grade 3-5 non-hematologic toxicities (≥4 vs. <4) using FisherÕs exact test. We further estimated progression-free survival (PFS) and overall survival (OS) using Kaplan-Meier methods.
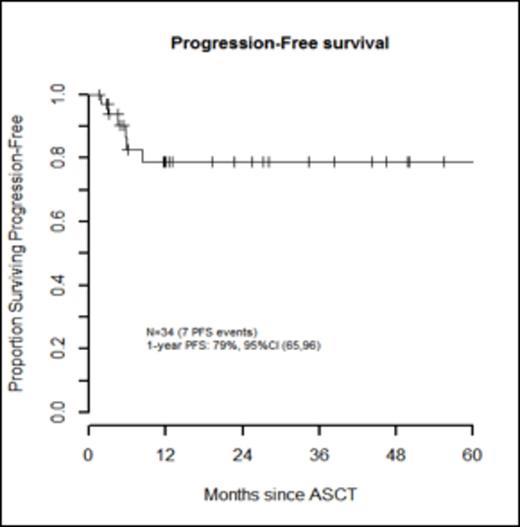
Results: Thirty-three patients (97%) experienced ≥ 1 grade 3-5 non-hematologic toxicity. Febrile neutropenia (grade 3) occurred in 32 pts (94%). Of all pre-HDT-ASCT variables, only the number of prior regimens (>2) was significantly associated with incurring more grade 3-5 non-hematologic toxicities, p=0.04 (Table 1). With a median follow-up for survivors of 12 months (range, 1.5-86.2 months), PFS was 79% (95% CI, 65-96) and OS was 82% (95% CI, 68-98) at 1 year (Figures 2 and 3). During the follow-up period, there were 7 pt deaths: 4 died of disease, 2 died secondary to TRM (5.9%), and one died of a secondary malignancy (squamous cell carcinoma) 86.2 months after HDT-ASCT. There were no progression events beyond 12 months. In a limited subset analysis wherein n=22 had first dose bu pharmacokinetics evaluated, pre-HDT-ASCT variables were not associated with higher bu AUC levels, though 64% of these pts required a dose reduction.
Conclusions: We reaffirmed that HDT-ASCT with TBC conditioning is effective consolidation for CNSL, but it is associated with more grade 3-5 non-hematologic toxicity in pts having had >2 prior regimens. Risk-adapted dose attenuation of TBC conditioning for this group of pts may mitigate observed toxicity.
Association of Pre-ASCT Variables & Grade 3-5 Non-hematologic Toxicities
| . | . | . | Number of Clinically Significant Grade 3-5 Toxicities . | . | |
|---|---|---|---|---|---|
| Pre-ASCT Variables | All (N=34) | Fewer than 4 (N=21) | 4 or more (N=13) | p-value | |
| Age | 0.71 | ||||
| <60 | 23 (68%) | 15 (71%) | 8 (62%) | ||
| ≥60 | 11 (32%) | 6 (29%) | 5 (38%) | ||
| Gender | 0.72 | ||||
| Female | 13 (38%) | 9 (43%) | 4 (31%) | ||
| Male | 21 (62%) | 12 (57%) | 9 (69%) | ||
| Disease | 0.30 | ||||
| PCNSL | 19 (56%) | 10 (48%) | 9 (69%) | ||
| SCNSL | 15 (44%) | 11 (52%) | 4 (31%) | ||
| KPS | 0.99 | ||||
| ≥80 | 32 (94%) | 20 (95%) | 12 (92%) | ||
| <80 | 2 (6%) | 1 (5%) | 1 (8%) | ||
| BMT HCT CI | 0.99 | ||||
| ≤2 | 17 (50%) | 11 (52%) | 6 (46%) | ||
| >2 | 17 (50%) | 10 (48%) | 7 (54%) | ||
| Number of Prior Regimens | 0.04 | ||||
| ≤2 | 21 (62%) | 16 (76%) | 5 (38%) | ||
| >2 | 13 (38%) | 5 (24%) | 8 (62%) | ||
| WBRT | 0.17 | ||||
| No | 28 (82%) | 19 (90%) | 9 (69%) | ||
| Yes | 6 (18%) | 2 (10%) | 4 (31%) | ||
| Disease state prior | 0.99 | ||||
| CR/CRu | 29 (85%) | 18 (86%) | 11 (85%) | ||
| PR | 5 (15%) | 3 (14%) | 2 (15%) | ||
| . | . | . | Number of Clinically Significant Grade 3-5 Toxicities . | . | |
|---|---|---|---|---|---|
| Pre-ASCT Variables | All (N=34) | Fewer than 4 (N=21) | 4 or more (N=13) | p-value | |
| Age | 0.71 | ||||
| <60 | 23 (68%) | 15 (71%) | 8 (62%) | ||
| ≥60 | 11 (32%) | 6 (29%) | 5 (38%) | ||
| Gender | 0.72 | ||||
| Female | 13 (38%) | 9 (43%) | 4 (31%) | ||
| Male | 21 (62%) | 12 (57%) | 9 (69%) | ||
| Disease | 0.30 | ||||
| PCNSL | 19 (56%) | 10 (48%) | 9 (69%) | ||
| SCNSL | 15 (44%) | 11 (52%) | 4 (31%) | ||
| KPS | 0.99 | ||||
| ≥80 | 32 (94%) | 20 (95%) | 12 (92%) | ||
| <80 | 2 (6%) | 1 (5%) | 1 (8%) | ||
| BMT HCT CI | 0.99 | ||||
| ≤2 | 17 (50%) | 11 (52%) | 6 (46%) | ||
| >2 | 17 (50%) | 10 (48%) | 7 (54%) | ||
| Number of Prior Regimens | 0.04 | ||||
| ≤2 | 21 (62%) | 16 (76%) | 5 (38%) | ||
| >2 | 13 (38%) | 5 (24%) | 8 (62%) | ||
| WBRT | 0.17 | ||||
| No | 28 (82%) | 19 (90%) | 9 (69%) | ||
| Yes | 6 (18%) | 2 (10%) | 4 (31%) | ||
| Disease state prior | 0.99 | ||||
| CR/CRu | 29 (85%) | 18 (86%) | 11 (85%) | ||
| PR | 5 (15%) | 3 (14%) | 2 (15%) | ||
Bhatt:Spectrum: Consultancy. Moskowitz:GSK: Research Funding; Merck: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding. Giralt:TAKEDA: Consultancy, Honoraria, Research Funding; JAZZ: Consultancy, Honoraria, Research Funding, Speakers Bureau; AMGEN: Consultancy, Research Funding; SANOFI: Consultancy, Honoraria, Research Funding; CELGENE: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal