Abstract
Background: The optimal approach for transplanting patients with primary immunodeficiency from a mismatched unrelated donor (MMUD) remains unclear. With reduced intensity conditioning, when bone marrow (BM) was used as the stem cell source in such patients we have previously observed a high rate of very low level donor chimerism (4 of 12 patients) requiring a second transplant procedure. The use of peripheral blood stem cells (PBSC) can overcome this but results in a high incidence of acute (≥ Gde II 10/21, Gde III-IV 5/21) and chronic (10/21, extensive 7/21) graft-versus-host disease (GVHD) (Rao et al in press). We hypothesized that the use of megadose CD34 selected stem cells from PB with addback of a T-cell dose akin to BM might facilitate engraftment through improved competition for the stem cell niche without severe GVHD.
Methods: We prospectively analysed outcomes on 20 consecutive patients with primary immunodeficiency (SCID n=5, HLH n=3, Other PID n=12) transplanted from 8/10 (n=4) or 9/10 (n=16) HLA MMUD at our institution between 2011-2015. The mean age at transplant was 5.1 years. All patients received reduced toxicity conditioning regimens (12/20 Fludarabine/Melphalan/Alemtuzumab; 8/20 Fludarabine/Treosulphan/Alemtuzumab) with Cyclosporin (CsA) and mycophenolate mofetil (MMF) GvHD prophylaxis. CD34 selection of donor PBSC was performed using CliniMACs and the mean CD34+ dose was 20.1x106/kg. At day 0 either 108 CD3/kg (cohort 1, n=6) or 3x108 CD3/kg (cohort 2, n=14) T-cells from the CD34- fraction were infused with the graft. Mean follow up was 31.9 months.
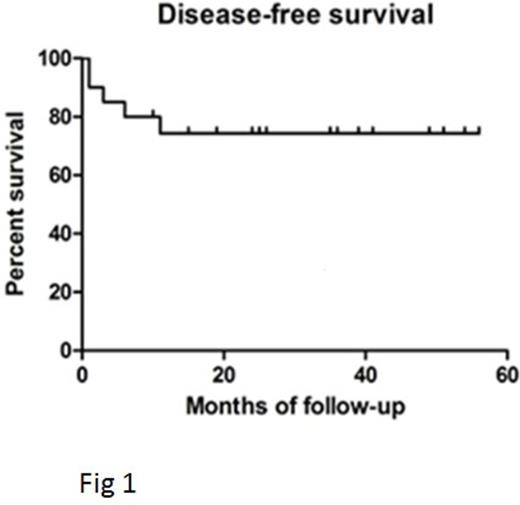
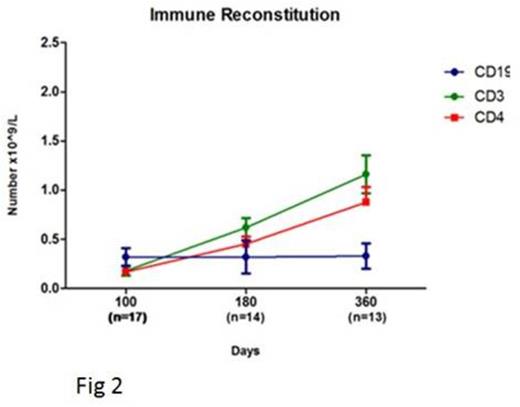
Results: In cohort 1, all patients engrafted and 2 developed high level donor mixed chimerism (both curative) in the myeloid and lymphoid lineages at last follow up. Two patients had Grade II acute GvHD and 1 had moderate chronic GvHD. In view of the slow immune reconstitution in this cohort, the T-cell addback dose was increased to 3x108/Kg in subsequent patients. In cohort 2, all patients achieved full donor haemopoiesis initially, 6/14 developed high levels of donor chimerism in both myeloid and lymphoid lineages later and 1/14 progressed with 10% donor T-cell engraftment but remains disease-free. The incidence of significant aGVHD was low (grade II n=4, no grade III or IV) and no patient developed cGvHD. Overall across both cohorts, 10 patients had viral reactivations and there were 5 deaths (3 viral complications, 1 pulmonary vasculopathy, 1 cGVHD lungs). The disease free survival at 2 years 7 months follow up was 70% which compares favorably with a previous cohort transplanted with unmanipulated BM as the stem cell source (Fig 1). Immune reconstitution was delayed (mean CD3 and CD19 counts at day 100 post-transplant was 245 x 109/L and 315 x 109/L) with similar kinetics in both cohorts (Fig 2), comparable to RIC transplant using unmanipulated BM. By 1 year post transplant 11/14 evaluable patients achieved normal CD3+T-cell numbers and 8/14 normal CD19+ B-cell counts.
Conclusion: The use of megadose peripheral blood stem cells with T-cell addback in the mismatched unrelated donor transplant setting results in high rates of curative engraftment and a low incidence of acute and chronic GvHD following reduced toxicity conditioning. However, T-cell reconstitution remains delayed (presumably reflecting the effect of Alemtuzumab on the infused T-cells) with a high incidence of viral complications.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal