Abstract
Introduction
The prognosis of multiple myeloma (MM) is heterogeneous and survival varies from a few months to more than 10 years. Numerous prognostic factors have been identified and several prognostic models were developed to stratify patients into various risk groups. Currently, the international staging system (ISS) is the most widely accepted prognostic model. However, since ISS was established using data collected from 1981 through 2002, only a small portion of patients treated with novel agents (e.g., thalidomide, bortezomib, and lenalidomide) were included in the analysis. Therefore its validity is being challenged in the era of novel agents. We aimed to develop an alternative prognostic model based on easily obtained clinical and laboratory data in the era of novel agents.
Patients and methods
Clinical and laboratory data were collected between January 2000 and April 2013 from 8 tertiary hospitals in Korea. Among 2893 newly diagnosed patients with MM, 1338 were treated with novel agents including thalidomide, bortezomib and lenalidomide at least once. Half of these 1338 patients were randomly selected as a training sample and the other half as a validation sample. A new prognostic model was developed in the training sample, based on significant adverse prognostic factors in univariate and multivariate analysis. This model was then validated in the validation sample.
Results
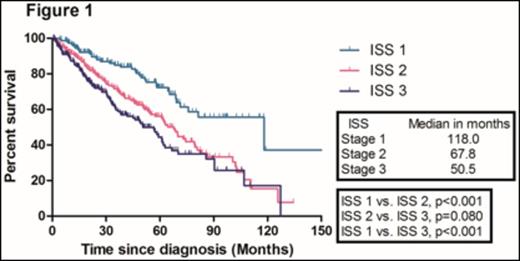
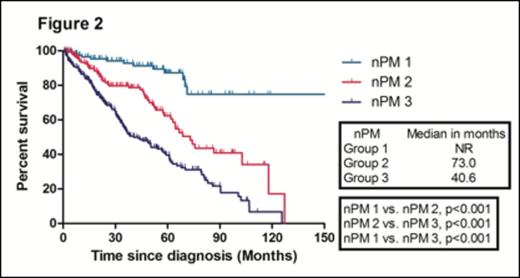
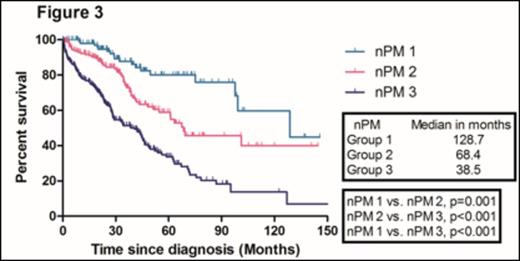
With a median follow-up duration of 50.8 months (range, 47.1- 54.5) in surviving patients, the median overall survival (OS) in the training sample (669 patients) was 67.8 months. The median OS of patients according to ISS 1, 2, and 3 were 118.0, 67.8, and 50.5 months, respectively. There were no significant difference in the median OS between ISS stage 2 and 3 patients (Figure 1). We identified four independent adverse prognostic factors in the training sample; Serum beta2-microglobulin (Sβ2M) ≥ 3.5 mg/L, elevated serum lactate dehydrogenase (LDH), Eastern Cooperative Oncology Group performance status (ECOG PS) ≥ 2 and bone marrow plasma cell percentage (BMPC) ≥ 60%. We used these four adverse prognostic factors to develop a new prognostic model and stratified the patients into three groups; Group 1, no adverse factor; Group 2, 1 adverse factor; Group 3, ≥ 2 adverse factors. We found that there were significant differences in median OS among these three groups (Not reached, 73.0, 40.6 months for group 1,2, and 3, respectively) (Figure 2). This new prognostic model was further validated in the validation sample (Median OS was 128.7, 68.4, and 38.5 months for group 1, 2, and 3, respectively) (Figure 3)
Conclusion
Our study suggests that this new prognostic model based on easily obtainable clinical and laboratory data (Sβ2M, LDH, ECOG PS, BMPC) might be a simple and useful tool to predict prognosis of patients with MM in the era of novel agents.
Kim:CELLTRION, Inc,: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal