Abstract
BACKGROUND: Immunomodulatory derivatives (IMiDs) have become an integral part of multiple myeloma (MM) treatment, both in newly diagnosed and relapsed refractory patients. Long-term treatment with IMiDs is inevitably associated with relapse and emerging resistance. The precise mechanisms of IMiDs resistance are not entirely clear. Gene-expression profiling (GEP) can be used to elucidate resistance/response signature associated with IMiDs.
PATIENTS AND METHODS: GEP of paired samples of CD138-purified plasma cells, obtained from patients prior to and 48 hours after single-agent therapy with thalidomide (42 newly diagnosed MM), lenalidomide (18 relapsed/refractory MM), or pomalidomide (18 relapsed/refractory MM) were compared. Samples were hybridized to Affymetrix expression arrays. Affymetrix U133Plus2.0 arrays were used in the pomalidomide study and Affymetrix U95Av2 arrays were used for thalidomide and lenalidomide pharmacogenomics studies. Among 176 genes that significantly changed pre- and post IMiD treatment (P<0.05 by SAM analysis, and with change in same direction), 14 genes were selected that had P values <0.05 based on univariate Cox regression analysis for PFS in thalidomide arm of TT 2 trial. These 14 genes were combined to create a continuous score based upon each patient's difference between the average log2-scale expression of the 4 prognosis-unfavorable (hazard ratio [HR] > 1) and the 10 prognosis-favorable (HR < 1) genes. An optimal cutoff for this IMiDs-based risk score (hereafter referred to as IMiD14) was then established with the running log-rank test, so that patients with scores higher than the cutoff were considered as IMiDs resistant patients.
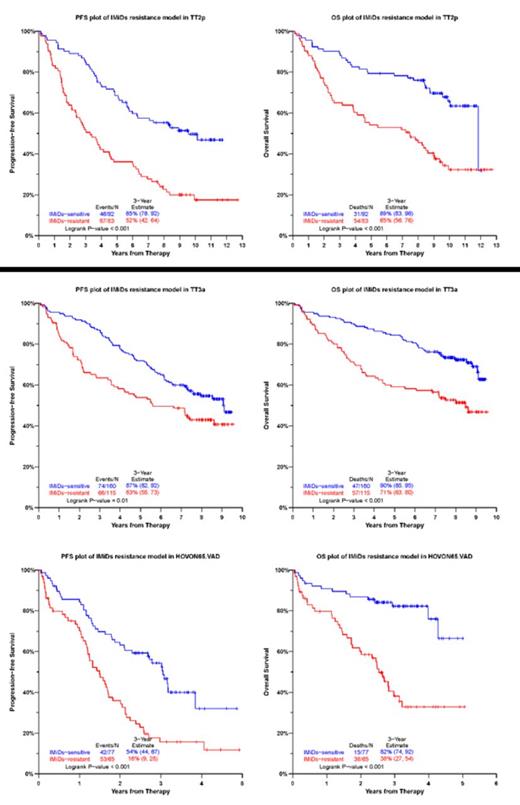
RESULTS: The IMiDs resistant group in TT2 (Thalidomide arm) training set exhibited significantly poor PFS (P < 0.001) and inferior OS (P < 0.001). Next, we examined whether IMiD14 model had outcome discriminatory power in several independent test cohorts treated with IMiDs. In validation cohort TT3a (induction with VT(thalidomide)D-PACE, and maintenance with VTD in the 1st year followed by TD for 2 years), there were significant differences in outcome between patients whom IMiD14 model predicted to have IMiDs resistance versus others, 3-year PFS and OS rates for the predicted IMiDs resistant patients were 63% and 71%, whereas for the rest of patients the corresponding rates were 87% and 90% (PFS P = 0.01, OS P < 0.001). In validation cohort TT3b (induction with VT(thalidomide)D-PACE, and maintenance with VRD for 3 years), 3-year PFS and OS rates for the predicted IMiDs resistant patients were 62% and 70%, whereas for the rest of patients the corresponding rates were 80% and 85% (PFS P = 0.002, OS P = 0.003). The discriminatory power of IMiD14 was also independently confirmed in the validation cohort HOVON65/GMMG-HD4 VAD arm (induction with vincristine, doxorubicin, and dexamethasone, and maintenance with thalidomide for 2 years); 3-year PFS and OS rates for the predicted IMiDs resistant patients were 16% and 38%, versus 54% and 82%, respectively for the rest of the patients (PFS P < 0.001, OS P < 0.001).
CONCLUSION: We have identified IMiD14 gene expression signature that accurately predicts resistance to IMiD treatment and outcome in multiple myeloma patients. These predictive genes could provide novel targets for therapeutic intervention and are being evaluated in prospective datasets.
Barlogie:Celgene: Honoraria, Research Funding; Millennium: Honoraria, Research Funding; Novartis: Consultancy. Sonneveld:Janssen-Cilag, Celgene, Onyx, Karyopharm: Honoraria, Research Funding; novartis: Honoraria. Usmani:Celgene: Honoraria, Speakers Bureau; Onyx: Honoraria, Research Funding, Speakers Bureau; Millennium: Honoraria, Speakers Bureau; Janssen Oncology: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Pharmacyclics: Research Funding; Array BioPharma: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal