Abstract
Background:
PRCA is characterized by severe normochromic normocytic anemia, reticulocytopenia, and markedly reduced bone marrow erythroid precursors with intact leukocytic and megakaryocytic lineages. There is limited information about the prevalence and outcomes of PRCA in CLL, with the vast majority of information derived from case reports and small series comprising <10 patients. Here, we describe the clinical features, therapy, and outcomes of CLL patients with PRCA seen at our institution.
Methods:
The Mayo Clinic CLL Database includes all patients with a diagnosis of CLL evaluated at Mayo Clinic Rochester, MN between January 1995 and December 2014. We identified patients diagnosed with PRCA per the following diagnostic criteria: those who presented with normocytic anemia, had decreased erythroid precursors on bone marrow examination, and reticulocytopenia that could not be explained by any other cause besides PRCA. The baseline demographics, clinical characteristics, therapy, and outcomes were abstracted from the electronic medical records. We generated descriptive statistics and calculated overall survival (OS) from diagnosis date (PRCA, autoimmune cytopenia [AID], or CLL) to last known alive or death date. OS was plotted using Kaplan Meier curves and compared between groups using log-rank statistic. The Mayo Clinic IRB approved this study.
Results:
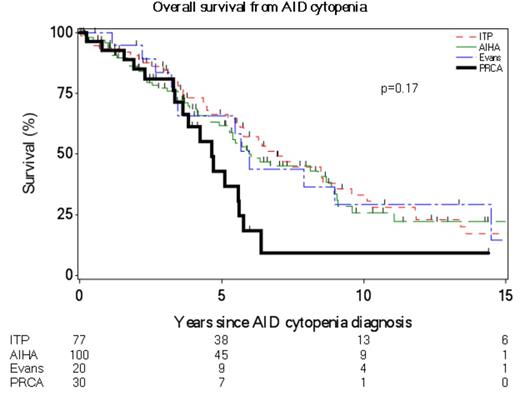
Of the 3945 CLL patients seen during the study interval, we identified 30 patients who met the diagnostic criteria of PRCA as described in the Methods section. The baseline characteristics, etiology and therapy for PRCA are shown in Table 1. The median time to PRCA from CLL diagnosis was 4.5 years (range-0.1-14 years). Of the 13 patients with IGHV results available, 12 (92%) were IGHV unmutated. Twenty-one (70%) were male and their median age at PRCA diagnosis was 67 years (range 40-81 years). Parvovirus testing was positive in 6 of 24 (25%) patients who were tested; 3 of these patients were treated with IVIG, 2 were treated with prednisone and IVIg, and 1 was treated with prednisone and rituximab. Eight patients (27%) had both PRCA and autoimmune hemolytic anemia (AIHA), and 1 patient (3%) had concomitant PRCA and immune thrombocytopenia (ITP). Of the 27 patients for whom we have treatment data, 10 required only one treatment regimen and 17 required 2-6 treatment regimens, with two patients requiring splenectomy for concomitant AIHA. The median survival of CLL patients after a diagnosis of PRCA was 4.6 years (Figure 1). There was no difference in survival between PRCA cases with and without parvovirus (p=0.92). Compared to newly diagnosed CLL Rai stage III/IV patients, there was no difference in OS (p=0.53). There was no statistically significant difference in survival from an AID cytopenia diagnosis when comparing PRCA, ITP, AIHA, and Evans syndrome (Figure 1; median 5 years for PRCA and 6 years for other groups; p=0.17).
Conclusion:
PRCA is a rare complication in CLL, occurring in <1% CLL patients seen at our institution over the past 20 years. Parvovirus was the etiologic factor in ~25% of patients and did not influence outcomes. There appears to be no significant difference in outcomes of PRCA patients compared to patients with other AID in CLL.
Patient Characteristics
| Characteristic . | N [range] or (%) . |
|---|---|
| Number of patients | 30 |
| Males | 21 (70) |
| IGHV status* · Unmutated · Mutated | 12 (92) 1 (8) |
| FISH results at CLL diagnosis* · 17p- · 11q- · Trisomy 12 · 13q- · Negative | 0 (0) 1 (10) 3 (30) 3 (30) 3 (30) |
| At PRCA Diagnosis | |
| Median age | 67 [40 - 81] |
| Median hemoglobin (gm/dL)* [normal range 12-15.5 gm/dL] | 8.2 [3.5 - 10.8] |
| Median absolute reticulocyte count (x 109/L)* [normal range: 38 - 113 x 109/L] | 3.5 [0.0 - 25.3] |
| Patients treated for CLL prior to PRCA diagnosis | 25 (83) |
| Etiology of PRCA | |
| Parvovirus (n = 24) | 6 (25) |
| Therapy-related: · Fludarabine · Alemtuzumab/rituximab · Pentostatin/cyclophosphamide/rituximab | 3 (10) 1 (3) 1 (3) |
| Autoimmune (neither therapy nor parvovirus related) | 19 (63) |
| Initial Therapy of Non-Parvovirus Related PRCA | |
| Steroids alone | 2 (8) |
| Steroids and Rituximab | 3 (13) |
| Steroids in combination with: · IVIG, Chlorambucil · Cyclosporine · Rituximab, IVIg · IVIg, splenectomy | 1 (4) 2 (8) 1 (4) 1 (4) |
| Monoclonal Ab alone · Alemtuzumab · Rituximab | 1 (4) 4 (17) |
| Cyclosporine | 1 (4) |
| Cyclophosphamide | 2 (8) |
| RCP (Rituxan, Cyclophosphamide, and prednisone) | 2 (8) |
| IVIg | 1 (4) |
| Not available | 3 (13) |
| Characteristic . | N [range] or (%) . |
|---|---|
| Number of patients | 30 |
| Males | 21 (70) |
| IGHV status* · Unmutated · Mutated | 12 (92) 1 (8) |
| FISH results at CLL diagnosis* · 17p- · 11q- · Trisomy 12 · 13q- · Negative | 0 (0) 1 (10) 3 (30) 3 (30) 3 (30) |
| At PRCA Diagnosis | |
| Median age | 67 [40 - 81] |
| Median hemoglobin (gm/dL)* [normal range 12-15.5 gm/dL] | 8.2 [3.5 - 10.8] |
| Median absolute reticulocyte count (x 109/L)* [normal range: 38 - 113 x 109/L] | 3.5 [0.0 - 25.3] |
| Patients treated for CLL prior to PRCA diagnosis | 25 (83) |
| Etiology of PRCA | |
| Parvovirus (n = 24) | 6 (25) |
| Therapy-related: · Fludarabine · Alemtuzumab/rituximab · Pentostatin/cyclophosphamide/rituximab | 3 (10) 1 (3) 1 (3) |
| Autoimmune (neither therapy nor parvovirus related) | 19 (63) |
| Initial Therapy of Non-Parvovirus Related PRCA | |
| Steroids alone | 2 (8) |
| Steroids and Rituximab | 3 (13) |
| Steroids in combination with: · IVIG, Chlorambucil · Cyclosporine · Rituximab, IVIg · IVIg, splenectomy | 1 (4) 2 (8) 1 (4) 1 (4) |
| Monoclonal Ab alone · Alemtuzumab · Rituximab | 1 (4) 4 (17) |
| Cyclosporine | 1 (4) |
| Cyclophosphamide | 2 (8) |
| RCP (Rituxan, Cyclophosphamide, and prednisone) | 2 (8) |
| IVIg | 1 (4) |
| Not available | 3 (13) |
*Not available for all patients
Ding:Merck: Research Funding. Kay:Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Research Funding; Hospira: Research Funding; Tolero Pharma: Research Funding; Celgene Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding. Shanafelt:Cephalon: Research Funding; Hospira: Research Funding; Glaxo-Smith-Kline: Research Funding; Genentech: Research Funding; Jannsen: Research Funding; Celgene: Research Funding; Polyphenon E International: Research Funding; Pharmacyclics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal