Abstract
BACKGROUND: MF is a myeloproliferative neoplasm characterized by bone marrow (BM) fibrosis, splenomegaly, and debilitating constitutional symptoms. RUX is a potent JAK1/JAK2 inhibitor that has demonstrated superiority in spleen volume reduction, symptom improvement, and survival in the phase 3 COMFORT studies compared with placebo and best available therapy. PAN, a potent pan-deacetylase inhibitor, inhibits JAK signaling by disrupting the interaction between JAK2 and heat shock protein 90, a protein chaperone. PAN has demonstrated reductions in splenomegaly and improvement of BM fibrosis in phase 1/2 studies. The combination of RUX and PAN demonstrated synergistic activity in preclinical MF models. Thus, a phase 1b study evaluating RUX plus PAN in pts with MF was initiated. We present results from the expansion phase of this study confirming the recommended phase 2 dose (RP2D) of RUX plus PAN combination therapy and its tolerability in pts with MF.
METHODS: Eligible pts had primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF classified as intermediate-1, -2, or high risk by International Prognostic Scoring System criteria and splenomegaly ≥ 5 cm by palpation. The primary objective was to determine the maximum tolerated dose and/or the RP2D of RUX-PAN combination therapy. Additional objectives included safety and efficacy. Exploratory endpoints included assessment of changes in BM fibrosis, JAK2 allele burden, and levels for 59 cytokines, with a focus on those known to be altered with RUX treatment. Pts received RUX 5-15 mg twice daily (bid) and PAN 10-25 mg 3 times weekly (tiw; days 2, 4, and 6) every other wk (qow) in a 28-day cycle. Following dose escalation and identification of the RP2D, additional pts were enrolled into the expansion phase and treated at this dose.
Results: At data cutoff (17 December 2014), 61 pts received treatment (escalation phase, n = 38; expansion phase, n = 23. Three dose-limiting toxicities were observed in the escalation phase (grade 4 thrombocytopenia, n = 2; grade 3 nausea, n = 1). The RP2D was confirmed to be RUX 15 mg bid and PAN 25 mg tiw qow. Among the 34 pts treated at the RP2D, 65% remained on treatment, and 21% discontinued due to adverse events (AEs); 65% had ≥ 1 dose interruption/change. The median duration of exposure to PAN and to RUX in these pts was 67.1 and 68.7 wk, respectively. The most common grade 3/4 hematologic AEs among pts treated at the RP2D, regardless of causality, were anemia (32%) and thrombocytopenia (29%); grade 3/4 nonhematologic AEs included diarrhea (18%), asthenia (12%), and fatigue (9%). Three deaths (due to progression of underlying disease, myocardial infarction, and hypoxic cardiac arrest) occurred on or within 30 days of treatment and were assessed by the treating investigator as unrelated to study treatment.
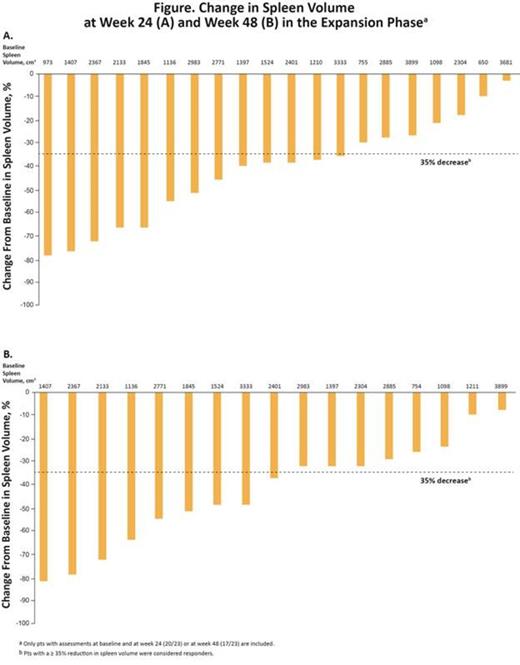
Most pts treated in the expansion phase had a reduction in spleen volume at wk 24 (87%; 20/23) and at wk 48 (74%; 17/23); 57% (13/23) and 39% (9/23) of pts achieved a ≥ 35% reduction from baseline in spleen volume at wk 24 and 48, respectively (Figure). Of 12 evaluable pts assessed for BM fibrosis grade by central review, 4 had improved fibrosis at wk 48, 6 had no change, and 2 worsened. Of the 17 pts in the expansion phase who were JAK2 V617F positive at baseline, 5 (29%) had a ≥ 20% decrease in allele burden by wk 48; most pts had a continuous decline in allele burden over time. Additionally, elevated levels of various markers of inflammation (IL-18, MMP-9, and MPO) normalized on treatment, whereas leptin levels increased, an effect that is associated with improvement in weight loss.
CONCLUSIONS: The combination of RUX and PAN was well tolerated and resulted in reductions in splenomegaly over the longer period of follow-up. 57% and 39% of pts achieved a spleen response at wk 24 and 48, respectively. Although no formal comparison can be made due to the small sample size of this study, combination therapy led to a higher proportion of pts achieving a spleen response vs ruxolitinib alone in the COMFORT studies. Reductions in JAK2 V617F allele burden and improvements in BM fibrosis were noted in some pts. Anemia, thrombocytopenia, and diarrhea were the most common AEs; AE rates were consistent with those observed with RUX and PAN monotherapies. Overall, the combination of RUX and PAN was associated with substantial treatment benefits in pts with MF and warrants further investigation through larger studies.
Harrison:Shire: Speakers Bureau; Sanofi: Honoraria, Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau; CTI Biopharma: Consultancy, Honoraria, Speakers Bureau; Gilead: Honoraria. Off Label Use: Ruxolitinib is a kinase inhibitor indicated for treatment of patients with intermediate or high-risk myelofibrosis, including primary myelofibrosis, post-polycythemia vera myelofibrosis and post-essential thrombocythemia myelofibrosis. Panobinostat is a histone deacetylase inhibitor indicated for the treatment of patients with multiple myeloma who have received at least 2 prior regimens . Kiladjian:Novartis: Consultancy; Incyte Corporation: Consultancy; Novartis: Other: Travel grant; Research Funding paid to institution (Hôpital Saint-Louis et Université Paris Diderot). Heidel:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Vannucchi:Novartis: Other: Research Funding paid to institution (University of Florence), Research Funding; Shire: Speakers Bureau; Baxalta: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Passamonti:Novartis: Consultancy, Honoraria, Speakers Bureau. Conneally:Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol-Myers Squibb: Honoraria, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Acharyya:Novartis Pharmaceuticals Corporation: Employment. Gopalakrishna:Novartis Pharma AG: Employment. Ide:Novartis: Employment, Equity Ownership. Liu:Novartis: Employment. Mu:Novartis: Employment. Ribrag:Esai: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmamar: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal