Abstract
Monitoring BCR-ABL1 transcript by quantitative PCR (qPCR) is essential for the management of CML patients treated with TKIs. Currently, up to 40-50% of CML patients treated with TKIs can achieve a deep molecular response (DMR = BCR-ABL1 minor or equal to0,01% IS), but only 50% of them are reported to maintain a stable Treatment Free Remission (TFR). Since qPCR has some intrinsic limitations in detection and quantification of BCR-ABL1 transcript, this method does not appear optimal in the selection of patients eligible for TKIs cessation. A precise monitoring of BCR-ABL1 transcript levels can help even better the clinicians in managing CML patients treated with TKIs and in selecting the best candidates for discontinuation of TKIs without relapse.
Digital PCR (dPCR) can be more advantageous than qPCR. It gives the absolute quantification of target nucleic acidsby partitioning the PCR reaction mix over a large number of wells, each containing a single copy or no copies of the target region. Based on the assumption of Poisson's distribution, the number of template copies originally presenting in the sample can be calculated from the number of partitions in which amplification has successfully occurred. In that way, standard curves cannot be necessary and data are more accurate.
In this study we set a dPCR assay to quantify the BCR-ABL1 transcript in a preliminary cohort of CML patients with major (MMR or MR3.0) or deep molecular response (MR4.0, MR4.5 and MR5.0).
The analysis by dPCR were based on a TaqMan-MGB probes targeting the BCR-ABL1 transcript. Custom assay was designed and produced with a FAM-label, basing on the sequence of routinely-used probes. The experiments were performed on a QuantStudio 3D Digital PCR System (Life Technologies). A commercial BCR-ABL1 Mbcr standard dilutions (Qiagen) and 10 replicates of blank samples (DNA-free, RNA-free water) were used to set dPCR assay and to determine the Limit of Detection and the Limit of Quantification of our method. The values of absolute quantities of BCR-ABL1 transcript assessed by dPCR were expressed as number of copies/ul. Samples for dPCR testing were obtained from peripheral blood (10 ml in EDTA tubes) of CML patients treated with TKIs, namely imatinib, nilotinib, or dasatinib, on time-checks planned for monitoring MMR or DMR through the conventional qPCR. To the purpose of the study, we evaluated 10 cases with stable MR3.0, 7 cases with stable MR4.0, 6 cases with stable MR4.5, 7 cases with stable MR5.0. It has been considered as stable any MR3.0, MR4.0, MR4.5, or MR5.0 molecular response measured by conventional qPCR and detected in the last three consecutive checks performed during the last 12 months. Blank samples served as negative controls, while 10 peripheral blood samples of healthy donors served as normal controls.
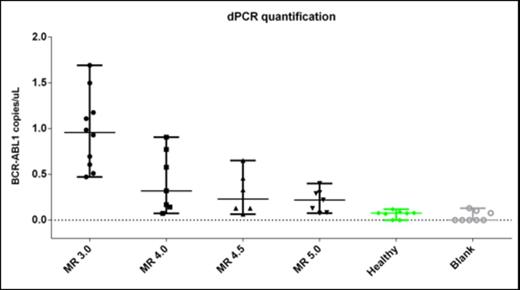
Digital PCR (dPCR) revealed different levels of BCR-ABL1 copies/µl among the CML patients achieving the major (MR3.0) or deep (MR4.0, MR4.5 AND MR5.0) molecular response with TKIs. Moving from MR3.0 to MR5.0 molecular response the median of BCR-ABL1 copies/µl assessed by dPCR were progressively decreasing (Figure 1), with blanks and healthy controls approximately to zero. Medians with ranges were 0.957 (0.472-1.692) for MR3.0; 0.319 (0.072-0.906) for MR4.0; 0.231 (0.063-0.651) for MR4.5; 0.219 (0.074-0.399) for MR5.0; 0.076 (0.000-0.118) for healthy controls, and 0.000 (0.000-0.129) for blanks. Moreover, dPCR revealed different BCR-ABL1 copies/µl among the patients of each class of molecular response. Importantly, in patients with MR4.5, MR5.0 and with undetectable levels of BCR-ABL1 % IS as measured with qPCR, discrete variable levels of BCR-ABL1 copies/µl have been detected by dPCR. These data, revealing different levels of BCR-ABL1 copies/µl beyond the limit of detection and quantification of conventional qPCR, may explain why no correlation was observed between BCR-ABL1 % IS levels measured by qPCR and numbers of BCR-ABL1 copies/µl measured by dPCR.
We are screening CML patients with MMR and DMR, but these preliminary results show that dPCR appears to be more accurate than qPCR for detection and quantification of BCR-ABL1 transcript and it should be seen as a useful step forward in order to better manage the TKI therapy and to better select the candidates for TFR.
Acknowledgments:
Department of Clinical and Experimental Sciences, University of Brescia; PRIN2009; European Leukaemia Net; BCC "Pompiano e Franciacorta".
Tiribelli:Bristol Myers Squibb: Consultancy, Speakers Bureau; Ariad Pharmaceuticals: Consultancy, Speakers Bureau; Novartis Farma: Consultancy, Speakers Bureau. Rosti:Bristol Myers Squibb: Honoraria, Research Funding, Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau. Martinelli:Roche: Consultancy; BMS: Speakers Bureau; MSD: Consultancy; ARIAD: Consultancy; Novartis: Speakers Bureau; Pfizer: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal