Abstract
Introduction: we previously reported (Vitolo U, JCO 2013) the results of a randomized study with brief first-line chemoimmunotherapy followed by rituximab maintenance vs observation. With a median follow-up of 42 months, 3-year Progression Free Survival (PFS) and Overall Survival (OS) were 66% and 89%, respectively. The addition of Rituximab maintenance gave a benefit to the patients: 2-year PFS was 81% for rituximab maintenance versus 69% for observation with a HR of 0.63 (95% CI: 0.38-1.05, p=0.079), although not statistically significant. Moreover we also found that achievement of Minimal Residual Disease (MRD) negativity predicted a better PFS: 3-year PFS 72% vs 39%, HR 3.1 (Ladetto M, Blood 2013). Overall these data showed the good efficacy of this brief chemoimmunotherapy regimen in elderly FL patients. Aim of this analysis was to report long-term outcome and long-term toxicities of this regimen.
Methods: From January 2004 to December 2007, 242 treatment-naive patients aged 60-75 years with FL Grade I, II and IIIa were enrolled by 33 FIL centres. Patients had to have advanced (high tumor burden stage II or stage III-IV) disease requiring treatment: 4 monthly courses of R-FND (standard doses of Rituximab, Fludarabine, Mitoxantrone, Dexamethasone) every 28 days followed by 4 weekly Rituximab infusions as consolidation. Responders patients [complete remission (CR) + unconfirmed CR + partial remission (PR)] were randomized to brief rituximab maintenance (Arm A), once every 2 months for a total of 4 doses, or observation (Arm B). MRD for the bcl-2/IgH translocation was determined on bone marrow cells in a centralized laboratory belonging to Euro-MRD consortium, using qualitative and quantitative PCR.
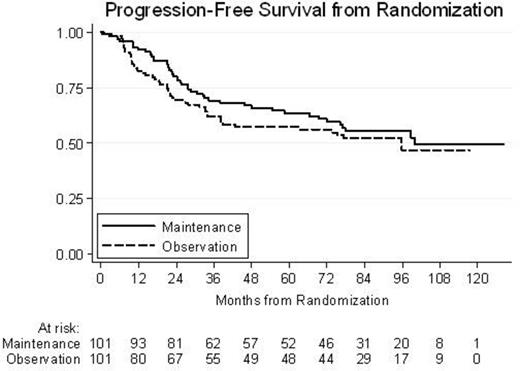
Results: a total of 234 patients began chemoimmunotherapy: after induction and consolidation treatment overall response rate was 86%, with 69% CR. Of these, 210 completed the planned treatment and 202 responders were randomized. Up to date, median follow-up were 96 months from enrollment and 87 months from randomization; additional follow-up data were available for 127/146 (87%) not relapsed/progressed patients. Five- and 7-year PFS for the whole population were 57% and 51%, respectively; 5- and 7-year OS for the whole population were 85% and 80%, respectively. From enrollment, an advantage in term of PFS and also OS was observed in FLIPI low risk patients: 7-year PFS was 67% for low risk versus 38% for intermediate-high risk patients (p<0.001) and 7-year OS was 86% versus 75%, respectively (p=0.03). After randomization, no differences between the two arms were detected for both PFS and for OS at 5 (data not showed) and 7 years: 7-year PFS was 55% for rituximab maintenance arm versus 52% for observation arm (p=0.331; HR 0.8); 7-year OS was 83% for both arms (p=0.208; HR 0.67). Moreover, after randomization no differences between the two arms were detected for both FLIPI low risk and intermediate-high risk patients: 7-year PFS was 67% for Rituximab maintenance arm versus 68% for observation arm (p=0.808) in low risk patients; in intermediate-high risk patients 7-year PFS was 46% vs 35% (p=0.301), respectively in Arm A vs B. Conversion to PCR negativity at the end of treatment maintains predictive value for better PFS: 7-year PFS were 58% and 36% (p=0.084), respectively for MRD negative vs positive patients. The same risk of late toxicity (infections or cardiac events) or secondary cancers was observed in both arms: in particular, 13 secondary neoplasms in maintenance arm vs 16 in observation arm were recorded.
Conclusions: the present long-term results of this trial with a prolonged follow-up of 7 years confirm that a good outcome is achievable in elderly FL patients with a short-term chemoimmunotherapy (R-FND + Rituximab consolidation) with a 7-year PFS of 51% and low toxicity. In addition these results did not show clear evidence in favor of a shortened Rituximab maintenance after R-fludarabine containing chemotherapy. Conversely, the achievement of PCR negativity maintains predictive value for a better outcome.
Off Label Use: Rituximab maintenance was not licensed in first-line treatment for follicular lymphoma at that time in Italy; Rituximab was provided free by Roche.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal