Abstract
Purpose: We assessed the survival outcome of patients with peripheral T-cell lymphoma-not otherwise specified (PTCL-NOS) and angioimmunoblastic T-cell lymphoma (AITL) who experienced disease progression or relapse after first line and subsequent therapy. We sought to evaluate the impact of recently approved drugs (pralatrexate and romidepsin) in these patients.
Patients and Methods: A total of 321 patients (180 PTCL-NOS, 141 AITL) initially diagnosed between 1999 and 2014 were retrospectively analyzed. Progression-free survival (PFS) and overall survival (OS) after the progression/relapse following first-line chemotherapy (PFS1 and OS1), after first salvage therapy (PFS2 and OSP2) and after second salvage therapy (PFS3 and OS3) were calculated. Outcome was separately analyzed according to the histopathologic subtype focusing on the use of new drugs.
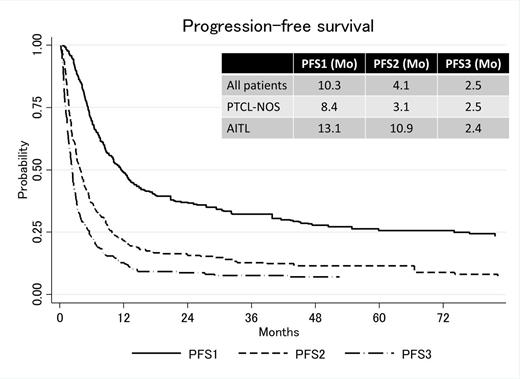
Results: The median age of the patients was 60 (range: 20-83). With a median follow up of 52 months, 240 patients (135 PTCL-NOS, 105 AITL) experienced progression/relapse after the first-line therapy, of whom 17 were post upfront stem cell transplant (SCT). A total of 54 patients received pralatrexate (n=41) and/or romidepsin (n=26); in 1st salvage in 9 and 17 patients, and after 2nd salvage in 17, 24 patients, respectively (13 patients received both). Thirty-three and 28 patients eventually underwent autologous and allogeneic SCT after salvage chemotherapy, respectively. Three patients received both auto and allogeneic SCT. In patients with PTCL-NOS, the median PFS1, PFS2 and PFS3 were 8.4, 3.1 and 2.5 months, respectively (Figure). The median OS1, OS2 and OS3 were 28.5, 10.9 and 7.0 months, respectively. In patients with AITL, the median PFS1, PFS2 and PFS3 were 13.1, 10.9 and 2.4 months, respectively (Figure). The median OS1, OS2 and OS3 were 47.7, 15.1 and 8.1 months, respectively. Use of pralatrexate or romidepsin at the 1st or after 2nd salvage therapy were not associated with longer PFS2 or PFS3, but, the patients who received pralatrexate at some point during therapy had significantly longer OS than who did not in patients with PTCL-NOS (median OS2: 8.5 vs 31.1 months), which however should be interpreted with caution as use of pralatrexate may be the consequence, not the cause, of longer survival. Multivariate analysis adjusting baseline PIT risk factors and the duration of the response to first line therapy revealed that use of romidepsin at any time during the treatment was associated with significantly longer OS2 in patients with PTCL-NOS (HR: 0.51, 95%CI: 0.28-0.93). Use of pralatrexate or romidepsin after relapse were not associated with longer PFS or OS in the patients with AITL.
Summary: Survival outcome for relapsed/refractory patients with relapsed/refractory PTCL-NOS and AITL remains poor. However, newer targetes agents may have potential to prolong survival in relapsed/refractory patients with PTCL-NOS. Caution is needed, however, in interpretation of this result due to the retrospective nature of the analysis. Further analyses are urgently needed to investigate the role of the use of newer agents at earlier lines of therapy to improve the survival outcome.
Westin:Spectrum: Research Funding. Nastoupil:Janssen: Research Funding; TG Therapeutics: Research Funding; AbbVie: Research Funding; Genentech: Honoraria; Celgene: Honoraria. Wang:Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal