Abstract
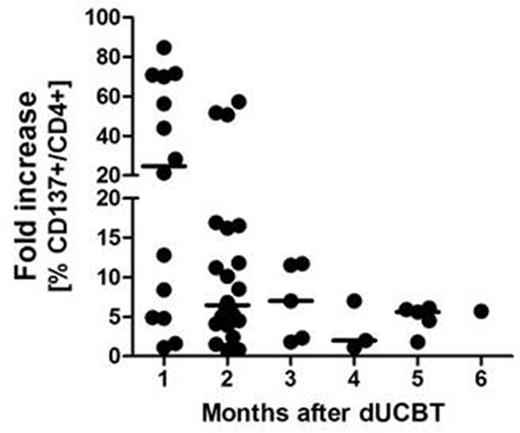
While dUCBT may be associated with less graft failure as compared to single UCBT, hematopoietic recovery following dUCBT generally originates from only a single graft, designated as the 'winning' graft. Graft predominance was suggested to be T-cell mediated, but is still incompletely understood. We recently showed that CD4+ T-cells rapidly expand after dUCBT and early CD4+ T-cell chimerism predicts for graft predominance (Somers et al BBMT 2012; Haematologica 2014). Given the frequent HLA class II allele mismatches between the 2 UCB units in dUCBT, we hypothesized that HLA class II-specific CD4+ T-cells from the 'winning' CBU may be responsible for rejection of the 'loser' CBU. In order to test that hypothesis, we evaluated whether 'wining' CD4+ T-cells specifically recognize individual HLA class II allele mismatches, expressed by the rejected graft. Patient T-cells were propagated in-vitro by 1. HLA unbiased polyclonal expansion (by K562 clone 2D11+CD3mAb and sequential addition of cytokines IL-7, Il-15 and IL-12), and 2. specific activation by HLA class II allele transduced HELA stimulator cells. In-vitro reactivity of propagated T cells was assessed in a co-culture with class II allele transduced HELA cells, and measured by analysis of T cell activation or effector markers by flow cytometry (FCM). 11 patients with poor-risk hematological malignancies were included, receiving 22 UCB units matched at HLA A, B, and DRB1 for 5/6 (n=7) or 4/6 (n=15) with the recipient. The median number (range) of class II allele mismatches between the 2 UCB units per transplant was 2 (1-6). In total, 33 different class II allele mismatches were tested, including 16 at HLA DRB1, 7 at DQB, and 10 at DP. Conditioning with TBI (4 Gy) combined with fludarabine and cyclophosphamide was applied and mycophenolate and ciclosporin were given for GVH-prophylaxis. All patients engrafted, at a median number of 26 days (range: 20-50) after transplantation (without G-CSF), and all developed complete single unit chimerism. Peripheral blood CD3+ T-cell numbers of samples taken at 1-6 months post UCBT were low (median: 0.207; range 0.030-0.699 x 10-9/L) with 74% (range, 8-96%) consisting of CD4+ T-cells. In all 11 patients, alloreactive CD4+ T-cells towards one or more mismatched class II alleles were detectable. In total, CD4+ alloreactivity towards 29 out of 33 (88%) mismatches was detected, including 15/16 for DRB1 (94%), 7/7 for DQ (100%), and 7/10 (70%) for DP alleles. All mismatched alleles but one (DPB1*04:01) elicited a CD4+ T-cell response. Stronger CD4+ T-cell reactivity was observed towards DRB1 and DQ. Analysis of activity towards matched, control alleles showed positivity in 2/11 (18%) combinations. Reactivity towards irrelevant third party alleles showed positivity in 3/17 (18%). The class II alloresponse was significantly higher for mismatched versus matched alleles (median fold increase over control: 7.4 (range 4.4-21.5) versus 1.4 (1.1-2.2), respectively). The highest alloreactive responses were observed in samples (n=14) taken at 1 month post UCBT (Figure). Alloreactive CD4+ T-cells upregulated CD137 and CD134, PD1 and the effector markers CD107 and Interferon-gamma. Collectively, these results demonstrate that specific effector alloreactivity by 'winner' CD4+ T-cells directed to multiple class II mismatched alleles was present in all patients, already at 1 month post UCBT. These results suggest that immediate cytotoxicity exerted by CD4+ T-cells from the 'winning' cord represent a novel mechanism of rapid rejection of the 'losing' unit after dUCBT following a non-ATG conditioning regimen. Furthermore, these observations suggest that the lower incidence of graft failure observed after dUCBT may, in part, result from an immunological, graft-potentiating effect evoked by mismatched class II alleles expressed by the rejected graft. Therefore, allele matching at HLA class II between the 2 units can be ignored in dUCBT. Class II mismatches between de UCB units might, alternatively, even be aimed for, but this should be confirmed in a prospective study. The potential graft versus leukemia effect of these alloreactive CD4+ T-cells is subject of ongoing investigation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal