Abstract
Introduction:
Despite intensive research most patients with acute myeloid leukemia (AML) still have a dismal prognosis. The transcription factor RUNX1 is a master regulator of myeloid differentiation and in AML its function is often disrupted by chromosomal translocations or mutations. These genetic alterations impact on leukemogenesis, disease progression and prognosis of AML patients. The expression of fusion proteins involving RUNX1 has been shown to exert a dominant-negative effect over wild-type RUNX1 leading to impaired myeloid differentiation and enhanced proliferation, while loss of RUNX1 may impact negatively on the frequency and self-renewal capacities of long-term hematopoietic stem cells. However, it remains unclear if differential expression of RUNX1 affects the AML phenotype. Here we analyzed the prognostic value of RUNX1 expression levels in AML patients undergoing allogeneic hematopoietic stem cell transplantation (HCT) after non-myeloablative conditioning (NMA).
Patients & Methods:
We analyzed 132 patients (median age 64 years [y], range 38-75y) with diagnostic bone marrow material available who received NMA-HCT (3x30mg/m2 Fludarabine on days -4 to -1 & 2Gy total body irradiation on day 0 followed by infusion of granulocyte-colony stimulating factor mobilized peripheral blood stem cells) at our institution between 2000 and 2012. Donors were human leukocyte (HLA)-matched related (15%) or HLA-matched (61%) or mismatched (24%) unrelated. 63 (48%) patients had a normal karyotype. Presence of FLT3 -ITD or FLT3 -TKD, mutational status of IDH1, IDH2, CEBPA, NPM1 and DNMT3A as well as expression levels of mir-9, mir-181a, BAALC, ERG and MN1 were determined at diagnosis. European LeukemiaNet cytogenetic classification was: 26% favorable, 27% intermediate-I, 20% intermediate-II and 27% adverse. RUNX1 expression at diagnosis was determined by qRT-PCR and normalized to 18S as internal standard. The median cut was used to define highand low RUNX1 expressers. Median follow-up was 3.9y for patients alive.
Results:
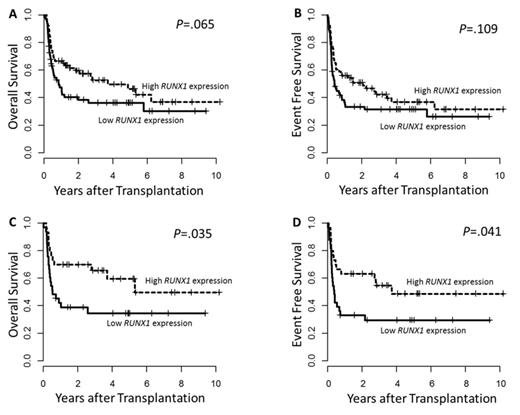
High RUNX1 expressers were more likely to have de novo AML (73% vs. 52%; P =.019) and higher % blasts in peripheral blood (median 38% vs. 19%; P =.004) and bone marrow (median 68% vs. 50%; P =.002) at diagnosis. Patients with high RUNX1 expression more often had IDH1 mutations by trend (P =.061) while there was no difference in IDH2 mutation frequency (P =.32). High RUNX1 expressers showed significantly higher ERG (P <.001), MN1 (P =.005) and mir-181a (P =.002) expression. High RUNX1 expression associated with longer overall (OS, P =.065) and event free survival (EFS, P =.109) by trend in the whole cohort (Figure 1 A, B). When we restricted our analysis to patients with a normal karyotype, high RUNX1 expression associated with a significantly longer OS (P =.035) and EFS (P =.041; Figure 1 C, D), while there was no prognostic impact of RUNX1 expression in patients with an abnormal karyotype (OS, P =.606 & EFS, P =.684). In multivariate analysis high RUNX1 expression independently associated with longer OS (Hazard Ratio 0.47 [95% Confidence Interval: 0.23 - 0.96]; P =.039) and longer EFS by trend (Hazard Ratio 0.57 [95% Confidence Interval: 0.29 - 1.14]; P =.113) in patients with normal karyotype.
Conclusion:
Our results revealed that high expression of the hematopoietic master regulator R UNX1 at diagnosis independently associated with survival in AML patients with normal karyotype receiving NMA-HCT. High RUNX1 expression associated with distinct clinical and molecular markers. Assessing pretreatment RUNX1 levels may help to refine risk stratification in AML patients undergoing NMA-HCT.
Franke:Novartis: Other: Travel Costs; MSD: Other: Travel Costs; BMS: Honoraria. Niederwieser:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal