Abstract
The activated B-cell-like (ABC) subtype of diffuse large B-cell lymphoma (DLBCL) is a very aggressive lymphoma characterized by constitutive NF-kB activation, but whether miRNAs dysfunction contributes to this event, and their exact function and mechanism remain unclear. Starting from an integrative screening strategy, we revealed that there were some interactions between the NF-kB signaling and miR-17~92 cluster, which was essential for B-cell development and commonly gained and/or overexpressed in ABC-DLBCL. Several important NF-kB negative regulators including TNFAIP3 (A20), CYLD and Rnf11 were predicted and validated to be the direct targets of miR-17~92. Conditional knock-down of miR-17~92 using sponge could suppress NF-kB activity and elevate the A20, CYLD and Rnf11 expression in 293T cells. Furthermore, we demonstrated that enforced overexpression of miR-17~92 could also decrease the A20, CYLD and Rnf11 expression in ABC-DLBCL cells. Conditional overexpression of miR-17~92 could promote ABC-DLBCL cells growth, accelerate the cells G1/G0 phase to S phase transition, and suppress NF-kB inhibitor-induced apoptosis. Conversely, conditional knock-down of miR-17~92 could inhibit ABC-DLBCL cells growth and sensitize the cells to NF-kB inhibitor-induced apoptosis. The miR-17~92 could induce the IkB-a and NF-kB p65 phosphorylation, leading to the NF-kB activation and aberrant expression of NF-kB transcriptional target genes. However, miR-17~92 did not regulate the NF-kB p52/p100 phosphorylation. Overexpression of miR-17~92 enhanced K63-linked ubiquitination and reduced K48-linked ubiquitination of the TNFa receptor 1 complex including RIP1. Importantly, we found that high expression level of miR-17~92 was associated with poorer survival in ABC-DLBCL patients. Our results uncovered a novel mechanism for the canonical but not the non-canonical transcription factor NF-kB pathway by modulation of miR-17~92 in ABC-DLBCL, and suggested that targeting the miR-17~92 might be novel bio-therapeutic strategies, which could be single-agent or combined with NF-kB inhibitor treatment, for ABC-DLBCL patients.
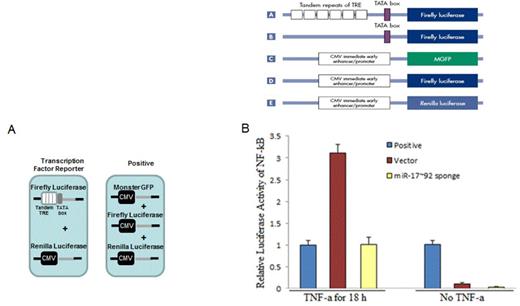
Inhibition of miR-17~92 blocks the activity of NF-kB in HER293T cells: (A) The schematic representation of reporter constructs involved in assay. (B) HEK293T cells were co-transfected with Dul-Luciferase reporter constructs and the miR-17~92 sponge plasmid. TNF-a stimulation or without stimulation for 18 h.
Inhibition of miR-17~92 blocks the activity of NF-kB in HER293T cells: (A) The schematic representation of reporter constructs involved in assay. (B) HEK293T cells were co-transfected with Dul-Luciferase reporter constructs and the miR-17~92 sponge plasmid. TNF-a stimulation or without stimulation for 18 h.
miR-17~92 directly regulates A20, CYLD and Rnf11 in ABC-DLBCL cells. (A) Fluorescence images of tranduced ABC-DLBCL cells. (B) Expression of sponge or miR-17~92 in tranduced ABC-DLBCL cells. (C) Inhibition of miR-17~92 increase the expression of A20, CYLD and Rnf11. Overexpression of miR-17~92 reduce the expression of A20, CYLD and Rnf11 in ABC-DLBCL cells.
miR-17~92 directly regulates A20, CYLD and Rnf11 in ABC-DLBCL cells. (A) Fluorescence images of tranduced ABC-DLBCL cells. (B) Expression of sponge or miR-17~92 in tranduced ABC-DLBCL cells. (C) Inhibition of miR-17~92 increase the expression of A20, CYLD and Rnf11. Overexpression of miR-17~92 reduce the expression of A20, CYLD and Rnf11 in ABC-DLBCL cells.
miR-17~92 modulate mediate NF-kB activity in ABC-DLBCL. (A) Immunoblot analysis of IkB-a, NF-kB p65, NF-kB p100/p52 and their phosphorylation. (B) Heat-map display of quantitative real-time RT-PCR measurements of six independent NF-kB transcriptional targets show significantly lower expression in sponge expressing cells and higher expression in miR-17~92 expressing cells.
miR-17~92 modulate mediate NF-kB activity in ABC-DLBCL. (A) Immunoblot analysis of IkB-a, NF-kB p65, NF-kB p100/p52 and their phosphorylation. (B) Heat-map display of quantitative real-time RT-PCR measurements of six independent NF-kB transcriptional targets show significantly lower expression in sponge expressing cells and higher expression in miR-17~92 expressing cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal