Abstract
Cold agglutinin disease (CAD) is an autoimmune hemolytic anemia (AIHA) characterized by the presence of autoantibodies (cold agglutinins) that bind red blood cells (RBCs) and activate the classical complement pathway (CP). We have previously shown in vitro that in contrast to C5 inhibition, inhibition of the CP specific protease C1s prevents complement opsonin deposition on cold agglutinin-sensitized RBCs and protects them from phagocytosis, underscoring the necessity to block upstream CP activity (Shi et al., Blood, 2014). Based on the strong scientific rationale and nonclinical data, a Phase 1 clinical trial for TNT009, a monoclonal antibody (mAb) inhibitor of C1s, has commenced at the Medical University of Vienna, Austria. Phase 1a consists of healthy volunteer cohorts in single- and multiple-ascending dose protocols for which interim study results will be presented. In the integrated protocol design of Phase 1b, TNT009 will be dosed in patients with diseases in which pathological CP activity has been implicated, including CAD, warm AIHA and additional non-hematologic indications.
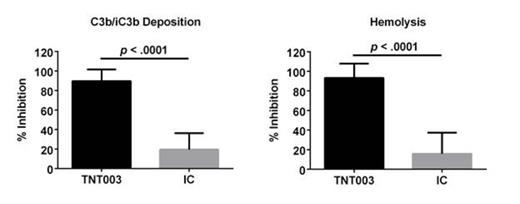
In anticipation of the clinical trial, we initiated a screening campaign in Vienna to find prospective CAD patients with serological markers of anemia and hemolysis. To date, plasma and serum samples have been collected from 15 CAD patients. Serum samples from 10 patients induce robust complement activation (C3b/iC3b deposition and/or hemolysis) on AET-treated human RBCs incubated in the patient's own complement-containing serum. In contrast to isotype control (IC), 100 mcg/mL of TNT003 (mouse parental mAb of TNT009), showed near complete inhibition of patient serum mediated C3b/iC3b deposition (90 ± 4%, n = 10; p< 1 x 10-5) and hemolysis (93 ± 5 %, n = 9; p< 1 x 10-5) (Fig. 1).
To further support the rationale of C1s inhibition in CAD, we asked whether serological signs of anemia and hemolysis were associated with evidence of increased in vivo CP activity in patient samples. We first examined how well experimental laboratory results agreed with standard clinical readouts. We found good concordance between patient sample induced C3 deposition on RBCs (FACS) and clinical C3 DAT scores (p< .05). Furthermore, IgM staining on RBCs incubated in patient samples (FACS) correlated well with cold agglutinin titers determined in the clinic (p < .001). Next, we observed that the extent of in vitro hemolysis correlated with C3d DAT scores (p< .05), LDH levels (p< .05), and bilirubin levels (p= .05). The agreement between the results from our in vitro patient sample-induced hemolysis assay with serologicalmarkers of complement activity, hemolysis and anemia used in the clinic suggest that our in vitro paradigm serves as a good model for in vivo complement activity in CAD patients. We then measured plasma C4 levels and CP activity in CAD serum samples (Wieslab Classical Pathway ELISA). We found that plasma C4 positively correlated with hemoglobin levels (p = .05). Additionally, we found an inverse correlation between serum CP activity and reticulocyte count (p < .05) and bilirubin levels (p = .05). These data demonstrate that in vivo consumption of the CP and its components (low CP activity, low C4) is associated with markers of anemia and hemolysis (low hemoglobin, high reticulocyte counts, high bilirubin).
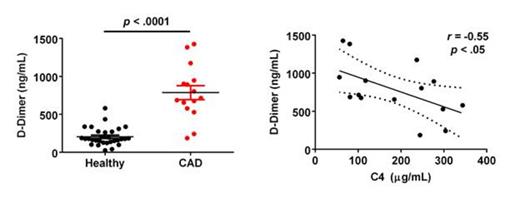
Finally, an emerging literature calls attention to an increased thromboembolic risk in AIHA, similar to that seen in patients with other hemolytic anemias such as paroxysmal nocturnal hemoglobinuria. We therefore measured D-dimer levels and found it significantly elevated in CAD patient plasma compared to healthy controls (p < .0001). Preliminary analyses show an inverse correlation of C4 and D-dimer in patient plasma (p < .05) suggesting that in vivo CP activity may contribute to the elevated thromboembolic risk in patients (Fig. 2). On-going analyses for other markers of thrombosis, in addition to other experimental approaches to assess the hypercoagulable state in these patients will seek to corroborate this finding.
The successful identification of CAD patients with altered complement and hematological profiles provides a unique opportunity to assess proof-of-concept early in the clinical development of TNT009.
TNT009 Parental mAb (TNT003) Inhibits CAD Serum Mediated Complement Activation on AET-Treated Human RBCs
TNT009 Parental mAb (TNT003) Inhibits CAD Serum Mediated Complement Activation on AET-Treated Human RBCs
Elevated D-dimer Correlates with Lower C4 Levels in CAD Patient Plasma
Jaeger:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; True North Therapeutics, Inc.: Research Funding; Hoffmann La Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Rose:True North Therapeutics, Inc.: Employment, Equity Ownership. Singh:True North Therapeutics, Inc.: Employment, Equity Ownership. Jilma:True North Therapeutics, Inc.: Consultancy, Research Funding. Gilbert:True North Therapeutics, Inc.: Employment, Equity Ownership. Panicker:True North Therapeutics, Inc.: Employment, Equity Ownership, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal