Abstract
Introduction: Hypercoagulability leading to thromboembolic complications is associated with significant morbidity and mortality in patients with colorectal cancer, however the underlying mechanisms are not well understood. Elevated levels of extracellular vesicles (EV), both microparticles (> 100 nm) and exosomes (< 100 nm), circulate in patients with colorectal cancer. While some studies suggest that EV-expressed tissue factor is associated with thrombosis in patients with cancer, overall results are inconsistent. However, EV contain many other mediators such as inflammatory cytokines and microRNAs (miRNA) that may be transferred to other cells and induce phenotypic changes. We hypothesized that EV derived from colorectal cancer cells in vitro would induce endothelial cell activation and promote endothelial cell procoagulant activity.
Methods: Human colorectal adenocarcinoma cells (HT-29) were cultured at a density of 1.0×107 cells per T75 flask in EV-free media containing 2% FBS, and after removal of floating cells and cell debris, microparticles were collected by centrifugation at 20,000 x G for 15 minutes; each flask contained approximately 4.0 x 106 annexin V+ microparticles. Exosomes were collected by additional centrifugation of the supernatant at 100,000 x G for 90 minutes. Microparticles and exosomes, at different dilutions, were separately co-cultured with confluent endothelial cell monolayers in 6 or 96-well plates, for 6 hours, using TNF-α (4 ng/ml) as a positive control. Endothelial cell activation was assessed by measuring expression of E-selectin, vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), Il-1β and tissue factor by quantitative RT-PCR (qPCR) and immunoblotting. We also analyzed the effects of microparticles and exosomes on endothelial cell procoagulant activity by layering plasma over confluent endothelial cell monolayers. Briefly, after incubating endothelial cell monolayers with microparticles or exosomes (or 4 ng/ml TNFα as a positive control) for 6 hours, monolayers were washed three times with 20mM HEPES buffer (pH 7.4) containing 5mM calcium chloride and then overlaid with 100 µl per well of pooled normal human plasma. Plasma was re-calcified by the addition of 11 ul 200 mM calcium chloride, and plates were then placed in a kinetic microtiter-plate reader (Synergy HT) maintained at 37°C, and fibrin formation measured as an increase in the optical density at 405 nm.
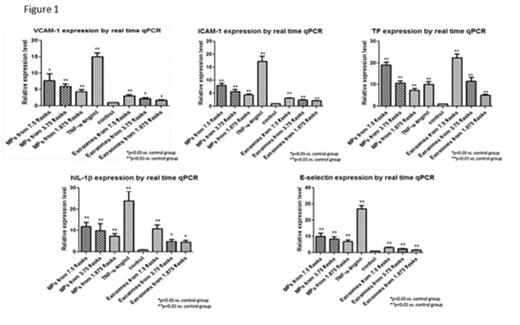
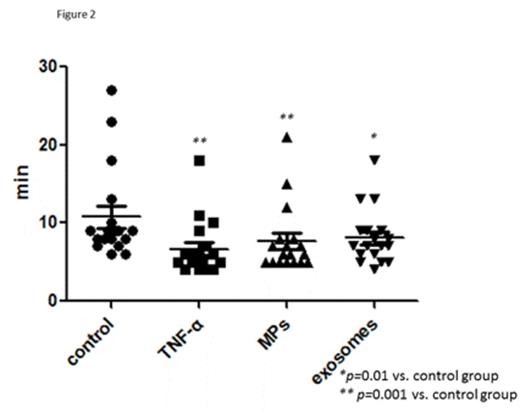
Results: Microparticles and exosomes constitutively released by HT-29 cells induced activation of endothelial cells in a concentration-dependent manner. Significant increases in the expression of E-selectin, ICAM-1, VCAM-1, tissue factor and IL-1β mRNA and protein were observed (Figure 1). In general, microparticles were more potent in inducing endothelial cell activation than exosomes when the EV were derived from the same number of HT-29 cells, though quantification of small exosomes is imprecise. Interestingly, while the response of endothelial cells to microparticles and exosomes was generally similar to that of 4 ng/ml TNFα, both types of EV appeared more potent in the induction of endothelial cell tissue factor, particularly at the mRNA level. HT-29 cell-derived EV, both microparticles and exosomes, also significantly shortened the clotting time of recalcified plasma added to endothelial cell monolayers (Figure 2).
Conclusions: EV constitutively released from HT-29 cells, both microparticles and exosomes, directly activate cultured endothelial cells leading to increased expression of cell adhesion molecules, elaboration of IL-1β, and expression of tissue factor. The change in endothelial phenotype is not due to simple transfer of biomolecules since EV stimulated changes at the mRNA and protein level. While responses to microparticles and exosomes were similar, some differences were observed, suggesting that different underlying mechanisms may contribute. Endothelial cell activation in response to EV leads to accelerated clotting of plasma on the endothelial cell monolayer. These findings suggest a novel effect of cancer cell-derived EV that may contribute to the hypercoagulability present in patients with malignancy.
Khorana:Daiichi Sankyo: Consultancy, Honoraria; Boehringer-Ingelheim: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Leo Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; sanofi: Consultancy, Honoraria. McCrae:Momenta: Consultancy; Halozyme: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Syntimmune: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal