Abstract
Background. Recent studies have described the landscape of recurrently mutated genes in mantle cell lymphoma (MCL), including genes involved in DNA damage response/cell cycle (ATM, TP53, CCND1), epigenetic regulation (KMT2D also known as MLL2, WHSC1), and cell signaling (BIRC3, TRAF2, NOTCH1). However, with the exception of TP53 abnormalities, little is known about the clinical relevance of recurrent mutations in MCL. Thus, we performed deep sequencing analysis of a MCL gene panel in the prospective series of patients enrolled in the ongoing FIL-MCL0208 phase III trial (EudraCTNumber: 2009-012807-25).
Patients and Methods. The study included untreated, advanced stage <65 years MCL patients that: i) were eligible into MCL0208 trial (R-CHOP followed by high-dose cytarabine, autologous stem cell transplantation, and randomization between lenalidomide maintenance vs observation); ii) were provided with baseline tumor material. A targeted resequencing gene panel including coding exons and splice sites of genes recurrently mutated in MCL (ATM, BIRC3, CCND1, KMT2D, TP53, TRAF2, WHSC1, NOTCH1) has been specifically designed. The gene panel allows the recovery of at least one mutation in >70% of cases. The gene panel was analyzed in tumor DNA from baseline bone marrow CD19+ purified MCL cells and, for comparative purposes to filter out polymorphisms, in the paired normal genomic DNA (available in 55% of cases) using a TruSeq Custom Amplicon target enrichment system followed by deep next generation sequencing (Illumina, median depth of coverage 2356x). Variants represented in >10% of the alleles were called with VarScan2 with the somatic function when the paired germline DNA was available. For patients lacking germline DNA, a bioinformatic pipeline including a number of stringent filters was applied to protect against the misclassification of polymorphisms as somatic variants. Primary endpoint of the analysis was progression free survival (PFS).
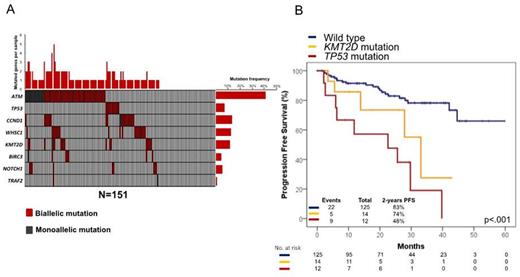
Results. Out of the enrolled patients, 151 are currently evaluable for mutations and clinical outcome (median age: 57 years, range 35-66; males 75%). Among prognostic factors, the MIPI was intermediate or high-risk in 49% of patients, the Ki67 ≥30% in 39%, and blastoid histology occurred in 8%. At the first planned interim analysis, median follow-up of alive patients was 26 months. At 2-years, 79% of patients were progression free and 91% alive (Cortelazzo et al EHA 2015). Overall, at least one mutation was detected in 106/151 cases (70%), including mutations of ATM in 42% of cases, CCND1 in 14%, WHSC1 in 13%, KMT2D in 12%, TP53 in 7%, NOTCH1 in 6%, BIRC3 in 5% and TRAF2 in 1% (Figure 1A). By univariate analysis, mutations of TP53 (2-years PFS 48% vs 82%; p<0.0001) and KMT2D (2-years PFS 67% vs 81%; p=0.004) associated with a significantly shorter PFS. By multivariate analysis, mutations of TP53 (HR: 4.39) and KMT2D (HR: 2.38) associated with an increase of the hazard of progression after adjusting for the MIPI. The hierarchical order of relevance in predicting PFS among molecular lesions was established by recursive partitioning analysis. TP53 mutations were the most predictive variable in the survival tree, followed by KMT2D mutations, thus providing the rationale to utilize these molecular features in the development of a model to predict PFS. By this approach, three MCL subgroups were hierarchically classified (Figure 1B). The high-risk category accounted for 8% of the cohort and included patients harboring TP53 mutations independent of the co-occurrence of KMT2D mutations (2-year PFS: 48%). The intermediate risk category accounted for 9% of the cohort and included patients harboring KMT2D mutations in the absence of TP53 mutations (2-year PFS: 74%). The low-risk category accounted for 83% of the cohort and comprised patients lacking both TP53 and KMT2D mutations (2-year PFS: 83%). The low number of events so far recorded prevented any analysis on overall survival.
Conclusions. Though limited by the short follow-up, our data show that: i) the combination of two genetic biomarkers (i.e. TP53 and KMT2D mutations) allows to predict the benefit that young MCL patients can gain from a cytarabine-based high dose sequential chemotherapy followed by autologous stem cell transplantation; ii) intensive chemotherapy does not overcome the negative prognostic impact of TP53 mutations; and iii) KMT2D mutations may represent a novel genetic biomarker in MCL patients.
Santoro:Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal