Abstract
Introduction
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare form of acute leukemia associated with an overall bad prognosis. Only very few cases have been reported to reach durable remissions thanks to chemotherapy alone. Allogeneic hematopoietic stem cell transplantation (HSCT) using a myelo-ablative conditioning regimen (MAC) has been reported to be the gold standard treatment for BPDCN (Roos-Weil et al, 2013). However, little is known about the place of reduced-intensity/non-myelo-ablative conditioning regimens (RIC/NMA) in this setting.
Methods
We retrospectively collected from the database of the French Society of Bone Marrow Transplantation and Cell Therapy (SFGM-TC) all cases of BPDCN treated with allogeneic HSCT. Immunophenotypes at diagnosis were centrally reviewed in order to confirm diagnosis according to the Garnache-Ottou diagnostic criteria (Garnache-Ottou et al, 2009). Twenty-eight patients had a diagnostic score of 2 or more. The remaining 15 patients all had CD4+ CD56+ disease, but as they were mostly diagnosed before publication of this score, other markers (such as CD123, BDCA-2 and BDCA-4) were not performed routinely at that time, precluding calculation of a score at least equal to 2.
Results
From February 2003 to January 2014, 43 patients with BPDCN received an allogeneic HSCT in 21 French centers. PatientsÕ characteristics are summarized in table 1. Median age was 57 (range: 20-72), sex ratio (M/F) was 2.1/1 and most patients were in CR1 at time of transplant. Sibling transplantation was performed in 42% of cases. Peripheral blood was the main source of stem cell used in this study (70% of cases). Conditioning regimens were MAC in 18 cases (42%) and RIC/NMA in 25 cases (58%, table 2).
Four patients (9%) had engraftment failure or secondary graft rejection, 3 of whom having received cord blood units. All these 4 patients were transplanted again 2 to 17 weeks after the first transplant.
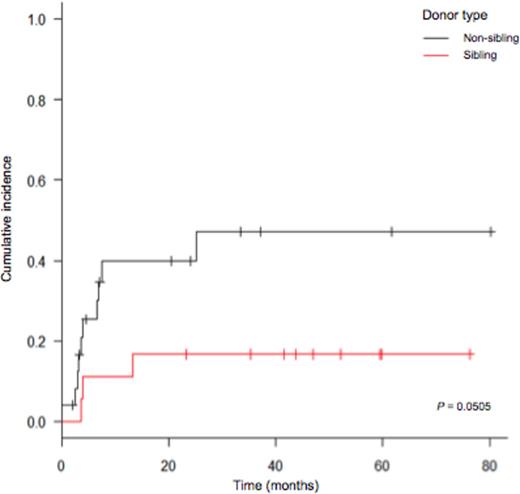
After a mean follow-up of 668 days for the entire cohort (1050 days for alive patients), 22 patients (51.2%) were alive, 19 of whom being disease-free (44.2%). Eleven patients had relapsed, at a median of 225 days post-HSCT (range: 74-821 days). Two-year cumulative incidences of relapse (CIR) and non-relapse mortality (NRM) were 25.5% (95% CI = [0.13-0.40]) and 32.8% (95% CI = [0.186-0.479]) respectively (figure 1). At 2 years post-transplant, disease-free survival (DFS) and overall survival (OS) were 44.9% (95% CI = [0.291-0.595]) and 52.2% (95% CI = [0.357-0.664]), respectively. Even though not statistically significant, patients receiving a MAC (n = 18) were less likely to relapse than patients receiving RIC/NMA (2-year CIR = 7.1% and 36% respectively, P = 0.137), but had a higher NRM rate (43.9% versus 26% at 2 years, P = 0.419), resulting in similar 2-year DFS and OS (57.1% versus 38%, P = 0.511 and 57.1% versus 49.7%, P = 0.91). There was a trend for a lower incidence of NRM at 2 years in patients transplanted from a sibling donor versus others (16.7% and 39.9% respectively, P = 0.0505, figure 2), but donor source had no effect on CIR (P = 0.826), DFS (P = 0.194) and OS (P = 0.188).
Conclusion
In this series of 43 patients with BPDCN, allogeneic HSCT was associated with a good disease control, but NRM was high. In this regard, transplantation from a sibling donor appears to be the best option. RIC/NMA are feasible and may also reduce the incidence of NRM, but at the expense of a higher incidence of relapse.
Patients' characteristics
| N . | 43 . | |
|---|---|---|
| Age | 57 (20-72) | |
| Sex (M/F) | 29/14 | |
| Time from diagnosis (days) | 170 (107-1050) | |
| Disease status at HSCT | ||
| CR1 | 34 (79%) | |
| CR2 | 5 (12%) | |
| No CR | 2 (5%) | |
| Unknown | 2 (5%) | |
| Donor | ||
| Sibling | 18 (42%) | |
| Unrelated | 23 (53%) | |
| Mismatch relative | 2 (5%) | |
| Cell source | ||
| Bone Marrow | 7 (16%) | |
| Peripheral Blood | 30 (70%) | |
| Cord Blood | 6 (14%) | |
| Conditioning regimen | ||
| MAC | 18 (42%) | |
| RIC/NMA | 25 (58%) | |
| CMV status (D/R) | ||
| -/- | 18 (42%) | |
| -/+ | 9 (21%) | |
| +/- | 4 (9%) | |
| +/+ | 12 (28%) | |
| GVHD prophylaxis | ||
| Ciclo/MTX | 15 (35%) | |
| Ciclo/MMF | 19 (44%) | |
| Ciclo alone | 5 (12%) | |
| Other | 2 (5%) | |
| Unknown | 2 (5%) | |
| N . | 43 . | |
|---|---|---|
| Age | 57 (20-72) | |
| Sex (M/F) | 29/14 | |
| Time from diagnosis (days) | 170 (107-1050) | |
| Disease status at HSCT | ||
| CR1 | 34 (79%) | |
| CR2 | 5 (12%) | |
| No CR | 2 (5%) | |
| Unknown | 2 (5%) | |
| Donor | ||
| Sibling | 18 (42%) | |
| Unrelated | 23 (53%) | |
| Mismatch relative | 2 (5%) | |
| Cell source | ||
| Bone Marrow | 7 (16%) | |
| Peripheral Blood | 30 (70%) | |
| Cord Blood | 6 (14%) | |
| Conditioning regimen | ||
| MAC | 18 (42%) | |
| RIC/NMA | 25 (58%) | |
| CMV status (D/R) | ||
| -/- | 18 (42%) | |
| -/+ | 9 (21%) | |
| +/- | 4 (9%) | |
| +/+ | 12 (28%) | |
| GVHD prophylaxis | ||
| Ciclo/MTX | 15 (35%) | |
| Ciclo/MMF | 19 (44%) | |
| Ciclo alone | 5 (12%) | |
| Other | 2 (5%) | |
| Unknown | 2 (5%) | |
Conditioning regimens
| MAC | 14 | |
| Cy/TBI | 11 | |
| Cy/TBI 12 Gy | 9 | |
| Cy/TBI 10 Gy | 1 | |
| Cy/Flu/TBI 12 Gy | 1 | |
| Bu/Cy | 3 | |
| RIC/NMA | 29 | |
| Flu/Bu/ALG | 10 | |
| Flu/TBI 2 Gy | 10 | |
| Flu/TBI 2 Gy | 5 | |
| Cy/Flu/TBI 2 Gy | 4 | |
| AraC/Flu/TBI 2 Gy | 1 | |
| Sequential | 5 | |
| Amsa/AraC/Flu/Cy/Bu/ALG | 3 | |
| Amsa/AraC/Flu/Cy/TBI 2 Gy/ALG | 1 | |
| Amsa/AraC/Flu/Bu/ALG | 1 | |
| Flu/Bu/Thiotepa/ALG | 1 | |
| Flu/Mel | 1 | |
| Cy/TBI 8 Gy | 1 | |
| TLI/ALG | 1 | |
| MAC | 14 | |
| Cy/TBI | 11 | |
| Cy/TBI 12 Gy | 9 | |
| Cy/TBI 10 Gy | 1 | |
| Cy/Flu/TBI 12 Gy | 1 | |
| Bu/Cy | 3 | |
| RIC/NMA | 29 | |
| Flu/Bu/ALG | 10 | |
| Flu/TBI 2 Gy | 10 | |
| Flu/TBI 2 Gy | 5 | |
| Cy/Flu/TBI 2 Gy | 4 | |
| AraC/Flu/TBI 2 Gy | 1 | |
| Sequential | 5 | |
| Amsa/AraC/Flu/Cy/Bu/ALG | 3 | |
| Amsa/AraC/Flu/Cy/TBI 2 Gy/ALG | 1 | |
| Amsa/AraC/Flu/Bu/ALG | 1 | |
| Flu/Bu/Thiotepa/ALG | 1 | |
| Flu/Mel | 1 | |
| Cy/TBI 8 Gy | 1 | |
| TLI/ALG | 1 | |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal