Abstract
Introduction: Busulfan (Bu) is a component of several conditioning regimens before hematopoietic stem cell transplant (HSCT). Bu has a narrow therapeutic window. High exposure was associated to higher rates of GVHD, VOD and mortality and low exposure to relapse or graft failure. Pharmacokinetic (PK) studies demonstrated high intra- and inter-patient variability. Several factors such as age, gender and weight have been associated to this variability (Vassal 1993, McCune 2009, Lee 2012, Veal 2012). The contribution of GSTA1 polymorphisms has been also documented. Haplotype *A2 was associated to higher clearance and less SCT-related complications, whereas haplotype *B (-69T) was associated to more frequent VOD occurrence (Ansari 2013). This study aims to analyse the influence of GSTA1 genotypes/haplotypes on Bu clearance.
Methods: Eighty-nine patients underwent a SCT after Bu-based conditioning with therapeutic drug monitoring (TDM) from October 2000 to August 2010 at our center CHU Sainte Justine, Montreal. The median age was 8.2 (IQR 1.6 to 13.9) years, 50.6% were males, most with malignancies (64.4%). Most patients received BuCy (77%) as conditioning regimen. Bu four times daily dose was given as previously described (Ansari 2014) and ulterior doses were calculated depending on the TDM. Bu AUC, steady-state concentration (Css) and Clearance were assessed after the first dose in all patients. New PK studies could be performed between sixth and ninth dose. Adjusted doses were calculated assuming a linear correlation between dose and desired AUC. Bu clearancewas expressed in base to an allometric representation of patients' size accounting for age, body weight and body composition: Normal Fat Mass (NFM). The NFM of 62Kg corresponds to an allometrically-scaled body weight of 70kg. Then, clearance was normalized by 62Kg NFM as described previously (McCune 2014). GSTA1 genotypes was classified as carriers of *A/*B haplotypes and their subtypes (Ansari 2013). Continuous data was expressed as median and interquartile range (IQR). A linear mixed model was performed to evaluate the association of normalized clearance to GSTA1 genotypes/haplotypes. Subjects were considered as random effects of repeated measures.
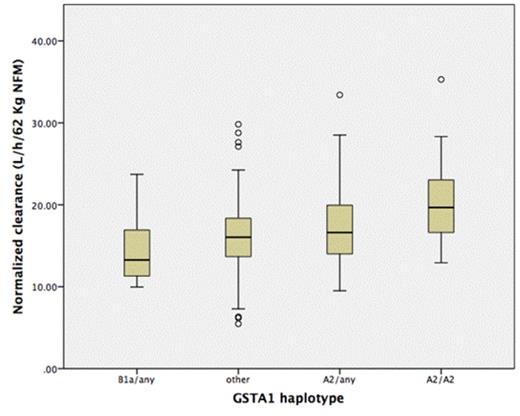
Results: Two hundreds and eighty PK measurements were performed (from one to 5 times per patient during the Bu exposure time). Median of Bu prescribed doses during the conditioning was 0.97mg/Kg. A TDM-based change of dose was possible from the 6th dose (30 hours after Bu initial dose). A dose change was made in 69.3% of patients. Median change was +32% (IQR 0 to +48.3%) of the initial dose. Eight percent of doses had to be decreased. Overall normalized clearance was 16.8 l/h/62 Kg NFM (IQR 13.7 to 19.4). Eighty-six percent were genotyped by GSTA1. In a model including age, gender and NFM normalized dose, clearance was significantly associated with GSTA1 haplotypes (p<0.001) as well as with age of transplantation and dose (both p<0.001). Haplotype GSTA1 * B1a (-1142G, -631G, -513C, -69T) carriers was significantly associated with lower clearances in comparison to other haplotypes (15.2L/h/62Kg NFM, 95% CI 13.6 to 16.8 versus 17L/h/62Kg NFM, 95% CI 16.5 to 17.4, p =0.03). Figure 1 shows the correlation between normalized clearance and GSTA1 haplotypes.
Conclusion: We have demonstrated that GSTA1 *B1a haplotype is an independent factor related to diminished Bu clearance. We suggest that GSTA1 genotyping should be included in the work-up before Bu-based conditioning regimens. Adjusted doses depending on *B1a status would limit the exposure to Bu in patients with lower rates of elimination and higher risk of HSCT-related complications, ensuring a more individualized and safer conditioning. However, it lacks Bu first dose prediction models accounting for genetics components such as GSTA1 haplotypes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal