Abstract
Background: The outcome for children with Burkitt lymphoma (BL)has improved significantly but for patients who relapse, the prognosis is dismal due to chemo-immunotherapy resistance (Cairo et al, JCO, 2012, Cairo et al, Blood, 2007). NK cells are bone marrow-derived cytotoxic lymphocytes that play a major role in the rejection of tumors. A variety of activating and inhibitory receptors on the NK cell surface are engaged to regulate NK cell activities and to discriminate target cells from other healthy 'self' cells. However, NK therapy is limited by several factors, including small numbers of active NK cells in unmodified peripheral blood, lack of tumor targeting specificity, and multiple mechanisms of tumor escape of NK cell immunosurveillance. Our group has successfully modified expanded peripheral blood Natural Killer cells (exPBNK) with an anti-CD20 CAR to target rituximab sensitive/resistant CD20+ BL cells in vitro and in NSG mice (Chu/Cairo, et al, Can Imm Res 2015). However, the short lifespan/persistence of adoptively transferred NK cells has limited the therapeutic efficacy. ALT-803 (Altor BioScience Corporation) is a superagonist of an IL-15 variant bound to an IL-15Rα-Fc fusion with enhanced IL-15 biological activity (Zhu et al. 2009 J Immunol), longer half-life and increased potency (Han, et al. Cytokine. 2011). It is currently in several clinical trials in patients with variety of cancers such as refractory indolent non-Hodgkin's lymphoma (NCT02384954).
Objective: We hypothesize that ALT-803, IL-15 superagonist complex, promotes exPBNK persistence and significantly enhances the cytotoxicity of anti-CD20 CAR exPBNK against CD20+ BL.
Method: PBMCs were expanded with lethally irradiated K562-mbIL21-41BBL cells (Dean Lee et al, PLoS One, 2012). CD56+ CD3- exPBNK cells were isolated using Miltenyi NK cell isolation kit. Anti-CD20-4-1BB-CD3 ζ mRNA (CAR mRNA) was producedin vitro and nucleofected into exPBNK as we have previously described (Chu/Cairo, et al, Can Imm Res 2015). ALT-803 was provided by Altor BioScience Corporation. ExPBNK cells were cultured with 0.35ng/ml or 3.5ng/ml ALT-803. NK proliferation was monitored with MTS assays. NK receptors expression and cytotoxicity were examined by flow cytometry (Chu/Cairo, et al, ASH 2014). NK resistant BL cells Raji and Daudi were used as target cells.
Results: % CD56+ CD3- PBNK cells were significantly increased compared to media alone at day 14 (mean 81.85% vs 14.91%, n=3, p<0.001) when co-cultured with the irradiated feeder cell K562-mbIL21-41BBL. The absolute NK numbers were enhanced with irradiated K562-mbIL21-41BBL cells as feeders compared to IL-2 alone after normalized to the INPUT NK cell numbers (mean 2247 fold±293.7 vs 0.516 fold±0.225, n=3, p<0.001) at day 14.
Different doses of ALT-803 or IgG were added to the culture medium of purified expanded exPBNK. Proliferation assays were performed at day 3, 7,11, and 17. ALT-803 significantly promoted exPBNK proliferation and persistence compared to IgG in vitro in a dose-dependent manner (A490 reading at 3.5ng/ml dose: ALT803 vs IgG=0.3383+0.009 vs 0.0987+0.0007, P<0.0001 at d17).
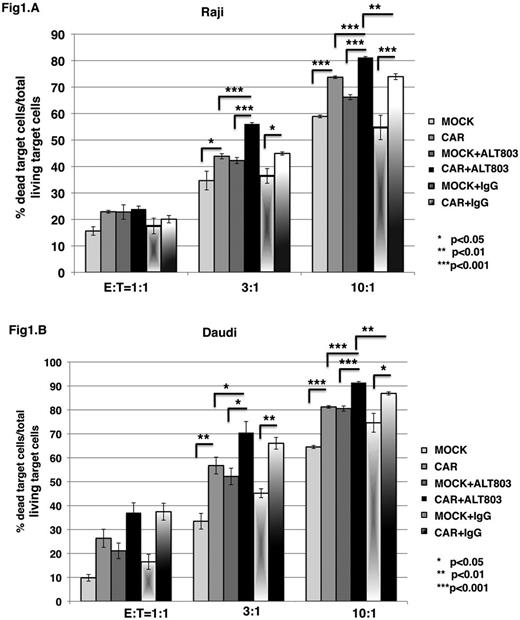
And ALT-803 significantly enhanced exPBNK cytotoxicity against NK resistant BL cells: Raji (ALT803 vs IgG= 49.54%+2.7% vs 5.99+0.34%, p<0.001, E:T=10:1) and Daudi (ALT803 vs IgG= 63.73%+3.09% vs 2.58+1.96%, p<0.001, E:T=10:1). It also maintained the highcytoxicity of exPBNK at d4, d10 and d18 against Raji (E:T=10:1, d4 vs d10 vs d18=62.07% vs 49.54% vs 61.47%) and against Daudi (E:T=10:1, d4 vs d10 vs d18=76.02% vs 63.73% vs 55%) by maintaining the activating receptors expression such as NKp30, NKp44, and NKp46.
Further-more, we demonstrated ALT-803 significantly enhanced the cytotoxicity of anti-CD20 CAR modified exPBNK against Raji (CAR vs MOCK= 81.19%+0.35% vs 66.19+0.94%, p<0.001, E:T=10:1) and Daudi (CAR vs MOCK= 91.41%+0.45% vs 80.56+1.07%, p<0.001, E:T=10:1) compared to mock modified exPBNK. ALT-803 also significantly enhanced the cytotoxicity of anti-CD20 CAR modified exPBNK against NK resistant BL cells: Raji and Daudi compared to anti-CD20 CAR modified exPBNK maintained in medium without ALT803 (Fig.1).
Conclusions: ALT-803 maintained the cytotoxicity of exPBNK and in vitro persistence and significantly enhanced anti-CD20 CAR exPBNK cytotoxicity against pediatric NK resistant BL. The in vivo effect of ALT-803 on CAR exPBNK using humanized NSG models is under investigation.
Wong:Altor BioScience Corporation: Employment, Other: stockholder of Altor Bioscience Corporation. Lee:Intrexon, Ziopharm, Cyto-Sen: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal