Abstract
Introduction
Recent results from the HELIOS phase 3 study in relapsed/refractory CLL/SLL demonstrated that the addition of ibrutinib to chemoimmunotherapy with bendamustine/rituximab (BR) leads to an 80% reduction in risk of progression or death compared with placebo + BR (Chanan-Khan et al. ASCO 2015). In addition to prolongation of progression-free survival (PFS; median not reached vs 13.3 months; hazard ratio: 0.203, 95% confidence interval: 0.150-0.276, p<0.0001), overall response rate (ORR) and rates of complete response (CR) or CR with incomplete marrow recovery (CRi) were significantly improved with ibrutinib + BR (ORR: 82.7% vs 67.8%; CR/CRi: 10.4% vs 2.8%). Here we report additional analyses on the quality of response with ibrutinib combined with BR.
Methods
In total, 578 patients (pts) with relapsed/refractory CLL/SLL requiring treatment were randomized 1:1 (289 per arm) to receive BR (≤ 6 cycles) with either ibrutinib (420 mg daily) or placebo. Purine analog refractoriness (yes vs no) and number of prior therapies (1 vs > 1) were stratification factors. Pts with deletion 17p (del17p; > 20% of cells) were excluded. All pts were required to have ≥ 1 abnormal lymph node (LN; defined as a measurable lesion > 1.5 cm). The primary end point was independent review committee (IRC)-assessed PFS. In this analysis, individual parameters of response (LN, spleen, overall radiology, absolute lymphocyte count [ALC], complete blood count [CBC], and bone marrow [BM]) were evaluated. Minimal residual disease (MRD) was assessed by flow cytometry using an 8-color panel (Rawstron AC, et al. Leukemia. 2007;21:956-964); MRD samples were collected at confirmation of suspected CR (bone marrow) and every 3 months thereafter (peripheral blood). Rate of MRD-negative response was defined as the proportion of pts who reached negative disease status (< 0.01%, ie, < 1 CLL cell/10,000 leukocytes) in any sample. Reported MRD rates are based on the intent-to-treat (ITT) population.
Results
The ORR assessed by the IRC was 82.7% with ibrutinib + BR vs 67.8% with placebo + BR (p < 0.0001), and was consistent with ORR reported by the treating physician (investigator assessed) (ORR: 86.2% vs 68.9%, p < 0.0001). However, rates of CR/CRi were higher by investigator assessment (21.4%, ibrutinib + BR vs 5.9%, placebo + BR) than IRC (10.4% vs 2.8%). The IRC employed an independent evaluation of radiological scans including stringent evaluation of LN and volumetric assessment of spleen size-the main reasons for the difference in CR/CRi rates between investigator and IRC assessment.
At baseline, similar proportions of pts in each arm had bulky disease defined as LN > 5 cm (54%-58%) or abnormal spleen (~40%) as assessed by IRC. Complete resolution of previous CLL/SLL manifestations as assessed by the IRC was achieved more often in the ibrutinib + BR arm in comparison with placebo + BR for LN (34.6% vs 15.2%), spleen (56.7% vs 37.0%), overall radiology (21.1% vs 9.0%), and BM (19.7% vs 5.9%). Rates of normalization of ALC (> 90%) and CBC (> 70%) were high and similar in the 2 arms.
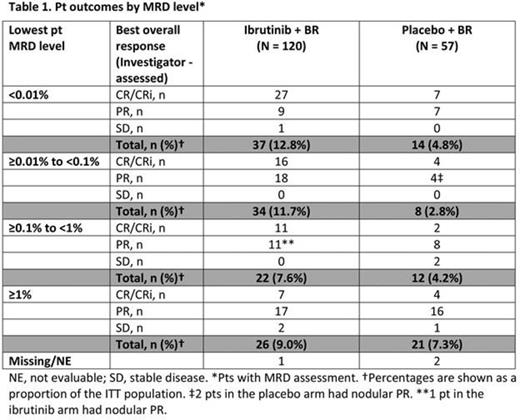
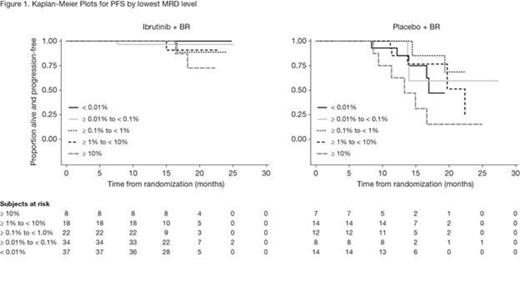
MRD was assessed in 120 pts treated with ibrutinib + BR and 57 pts treated with placebo + BR and was negative in 37 pts and 14 pts, respectively, which corresponds with an MRD-negative response rate of 12.8% vs 4.8% for ibrutinib + BR vs placebo + BR (p = 0.0011). Investigator-determined responses by different levels of MRD are shown in Table 1. The percentage of pts with an MRD level < 1% was higher with ibrutinib + BR vs placebo + BR (32.2% vs 11.8%). Moreover, the percentage of pts with an MRD level < 0.1% was higher for ibrutinib + BR vs placebo + BR (24.6% vs 7.6%) suggesting that the depth of response with ibrutinib + BR was superior. Kaplan-Meier plots by MRD level (Figure 1) show that a lower MRD level was associated with a longer PFS. However, pts in the ibrutinib + BR arm had a more sustained response at every MRD level compared with pts in the placebo + BR arm.
Conclusions
Pts treated with ibrutinib + BR showed not only a higher ORR, but also a greater depth of response, with more CRs and a higher rate of resolution of LN, spleen, and BM involvement. Furthermore, the rate and depth of MRD negativity was improved, and appeared to last longer in pts treated with ibrutinib + BR compared with pts receiving placebo + BR. With a median follow up of 17 months in the ibrutinib + BR arm, no MRD-negative pts and few MRD-positive pts had progressed, thus evaluating a trend in PFS with MRD negativity is currently limited in this arm.
Cramer:Gilead: Other: Travel grant, Research Funding; Glaxo Smith Klein/Novartis: Research Funding; Astellas: Other: Travel grant; Mundipharma: Other: Travel grant; Janssen: Other: Travel grant, Research Funding, Speakers Bureau; Hoffman LaRoche: Other: Travel grant, Research Funding, Speakers Bureau. Off Label Use: Combination of bendamustine, obinutuzumab and ibrutinib for treatment of CLL. Fraser:Hoffman LaRoche: Consultancy, Honoraria; Janssen: Honoraria, Research Funding, Speakers Bureau; Celgene: Honoraria, Research Funding. Demirkan:Amgen: Consultancy; Celgene: Other: Travel reimbursement. Santucci Silva:Merck: Research Funding; Celgene: Research Funding; GSK: Research Funding; Janssen: Other: Travel reimbursement, Research Funding; Hoffman LaRoche: Other: Travel reimbursement, Research Funding; Novartis: Other: Travel reimbursement. Janssens:Mundipharma: Speakers Bureau; Roche: Consultancy, Speakers Bureau; Janssen: Consultancy. Goy:Allos, Biogen Idec, Celgene, Genentech, and Millennium. Gilead: Speakers Bureau; Celgene: Consultancy, Research Funding, Speakers Bureau. Mayer:Janssen: Research Funding. Dilhuydy:Roche: Honoraria, Other: Travel reimbursement; Janssen: Honoraria, Other: Travel reimbursement; Mundipharma: Honoraria. Bartlett:Millenium: Research Funding; Medimmune: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; Genentech: Research Funding; ImaginAB: Research Funding; Astra Zeneca: Research Funding; Pharmacyclics: Research Funding; Janssen: Research Funding; Gilead: Consultancy; Seattle Genetics: Consultancy, Research Funding; Celgene: Research Funding. Rule:Roche: Consultancy, Other: Travel reimbursement; J&J: Consultancy, Other: Travel reimbursement, Research Funding; Celgene: Consultancy, Other: Travel reimbursement; Gilead: Research Funding. Sun:Janssen/J&J: Employment, Equity Ownership. Phelps:Janssen/J&J: Employment, Equity Ownership. Mahler:Janssen: Employment, Other: Travel reimbursement. Salman:Janssen/J&J: Employment, Equity Ownership. Schaffer:Janssen: Employment. Balasubramanian:Pharmacyclics LLC, an AbbVie Company: Equity Ownership; Janssen: Employment, Equity Ownership. Howes:Janssen/J&J: Employment, Equity Ownership. Hallek:AbbVie: Honoraria, Other: Speakers Bureau and/or Advisory Boards, Research Funding; Celgene: Honoraria, Other: Speakers Bureau and/or Advisory Boards, Research Funding; Pharmacyclics: Honoraria, Other: Speakers Bureau and/or Advisory Boards, Research Funding; Gilead: Honoraria, Other: Speakers Bureau and/or Advisory Boards, Research Funding; Boehringher Ingelheim: Honoraria, Other: Speakers Bureau and/or Advisory Boards; Roche: Honoraria, Other: Speakers Bureau and/or Advisory Boards, Research Funding; Mundipharma: Honoraria, Other: Speakers Bureau and/or Advisory Boards, Research Funding; Janssen: Honoraria, Other: Speakers Bureau and/or Advisory Boards, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal