Abstract
Introduction: The Food and Drug Administration (FDA) approved ibrutinib in February 2014 based on results from a pivotal phase 2 clinical trial that enrolled 85 patients with relapsed/refractory CLL. A subsequent phase 3 trial confirmed the superior progression-free survival (PFS) and overall survival (OS) of ibrutinib compared to ofatumumab in 391 CLL patients. However, there is no information on the efficacy and safety of ibrutinib in CLL patients treated outside the context of clinical trials. Additionally, it is unclear if the reasons for discontinuation of ibrutinib differ in routine clinical practice from those described in patients treated on clinical trials. We conducted a retrospective study to evaluate these aspects in a large cohort of CLL patients treated with ibrutinib off-protocol.
Methods: After IRB approval, CLL patients seen at Mayo Clinic, Rochester, MN, and treated with commercial ibrutinib from November 2013 through July 2015 were evaluated. The baseline characteristics, time to therapy discontinuation, reasons for discontinuation and survival following discontinuation were abstracted.
Results: One hundred thirty-five patients with CLL were treated during the study interval; 124 received ibrutinib for relapsed/refractory CLL and 11 received ibrutinib for treatment-naïve CLL with TP53 disruption (either del17p on FISH or TP53 mutation).
Patients with relapsed/refractory CLL (n=124)
Patients were high-risk, with a median of 3 prior therapies (range, 1-15), 81% had unmutated IGHV, and 38% had either del11q or del17p on FISH testing. After a median follow-up of 6.4 months, 101 remain on ibrutinib therapy. The estimated proportion of patients continuing ibrutinib at 6 months was 84% (95% CI: 77-92%) and at 12 months was 70% (95% CI: 59-83%).
Of the 23 who discontinued ibrutinib therapy, 8 discontinued for progressive disease (2 progressive CLL; 6 Richter's transformation). The remaining 15 discontinued therapy for reasons other than progression, including: infection (n=3), bleeding (n=3), diarrhea (n=1), atrial fibrillation (n=1), rash (n=1), immune thrombocytopenia (n=1), renal dysfunction (n=1), severe edema (n=1), MDS (n=1) and physician preference (n=2). Table 1 shows the reasons for discontinuation of ibrutinib in patients treated on clinical trials (Maddocks, JAMA Oncology, 2015) compared to the current cohort of patients.
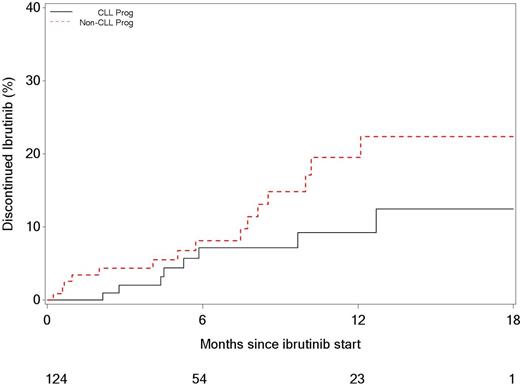
The estimated cumulative incidence of discontinuation of ibrutinib therapy due to CLL progression/Richter's transformation at 6 and 12 months was 9% (95% CI: 4-17%) and 12% (95% CI: 5-23%), respectively. The estimated cumulative incidence of ibrutinib discontinuation due to reasons other than progression at 6 and 12 months was 10% (95% CI: 5-17%) and 22% (12-34%), respectively (Figure).
The median survival after discontinuation of ibrutinib for patients who had CLL progression was 3.5 months, not reached in patients who developed Richter's transformation and not reached in those patients who discontinued ibrutinib due to reasons other than progression.
Patients with treatment-naïve CLL (n=11)
All patients had evidence of TP53 disruption (9 had del17p, 3 had TP53 mutation, and 1 had both). After a median follow-up of 2.8 months, no patient has had progression of CLL, and 2 patients have discontinued ibrutinib therapy for reasons other than CLL progression (atrial fibrillation, n=2). The estimated proportion of patients continuing ibrutinib at 6-months was 71% (95% CI: 45-100%). All 11 are still alive, and no patient has undergone allogeneic stem cell transplantation.
Conclusion: This is the first study that describes the outcomes of a large cohort of CLL patients treated with ibrutinib outside the context of clinical trials. Patients treated in routine practice appear more likely to discontinue ibrutinib for reasons other than progression of disease, compared to those participating in clinical trials. Our results confirm the poor survival of CLL patients who progress on ibrutinib.
Comparative analysis of reasons for discontinuation of ibrutinib in patients with relapsed/refractory CLL:
| Characteristic . | Current Study (%) . | Maddocks (JAMA Oncology 2015) (%) . | |
|---|---|---|---|
| No. of patients | 124 | 308 | |
| Median follow-up (months) | 6.4 | 20 | |
| Reasons for Discontinuation | |||
| Progression of disease | CLL | 2 (2) | 13 (4) |
| Richter's transformation | 6 (5) | 15 (5) | |
| Non-progression of disease | Infection | 3 (2) | 28 (9) |
| Other | 12 (10) | 17 (5) | |
| Characteristic . | Current Study (%) . | Maddocks (JAMA Oncology 2015) (%) . | |
|---|---|---|---|
| No. of patients | 124 | 308 | |
| Median follow-up (months) | 6.4 | 20 | |
| Reasons for Discontinuation | |||
| Progression of disease | CLL | 2 (2) | 13 (4) |
| Richter's transformation | 6 (5) | 15 (5) | |
| Non-progression of disease | Infection | 3 (2) | 28 (9) |
| Other | 12 (10) | 17 (5) | |
Ding:Merek: Research Funding. Kay:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; Hospira: Research Funding; Genentech: Research Funding; Pharmacyclics: Research Funding; Tolero Pharm: Research Funding. Shanafelt:Cephalon: Research Funding; Celgene: Research Funding; Glaxo-Smith_Kline: Research Funding; Polyphenon E Int'l: Research Funding; Hospira: Research Funding; Janssen: Research Funding; Pharmactckucs: Research Funding; Genentech: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal