Abstract
BACKGROUND
The dynamic relationship between HSC, their niches and the trabecular bone is disrupted in MPN as primary myelofibrosis (PMF).
PMF is associated to extramedullary hematopoiesis (EMH) usually with splenomegaly. Several tracers are used in nuclear medicine to assess EMH and bone marrow (BM) activity. Colloids labelled with Technetium 99m (99mTc) show the reticulo-endothelial system (RES) and Indium111 chloride (111In-Cl3) transferrin scintigraphy reflects the hematopoietic activity. Cell imaging using single photon emission tomography (SPECT) utilizes gamma emitting radiopharmaceuticals and 3D acquisition allowing for imaging planes reconstruction. It is often coupled to a computed tomography (CT) (SPECT/CT).
Few data exist about hematopoiesis assessment in PMF using hybrid imaging (HIm). We have shown its interest in EMH diagnosis. In PMF, we looked at patterns of active/inactive hematopoiesis in order to study later the impact of targeted therapies.
MATERIAL & METHODS
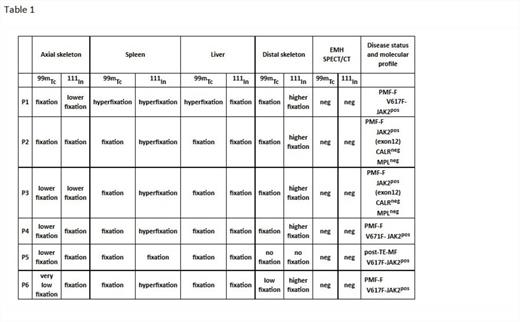
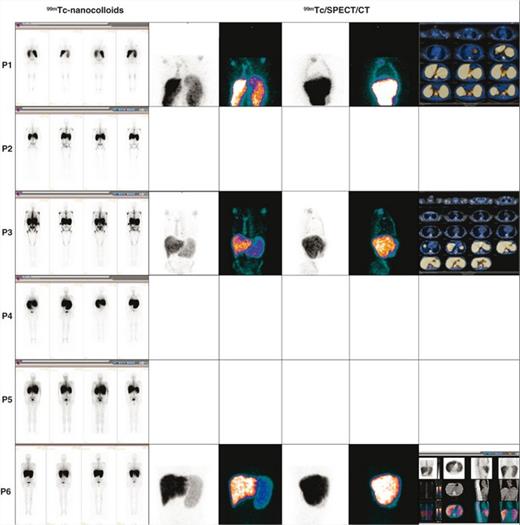
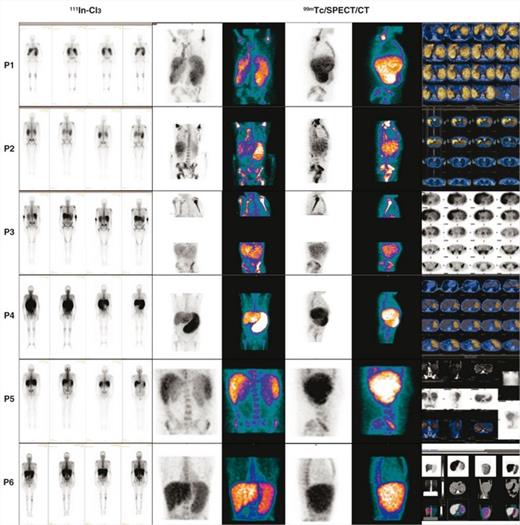
We performed HIm with 99mTc nanocolloids and 111In-Cl3 (coupled to SPECT/CT) in 5 untreated PMF patients and in 1 with essential thrombocytemia (ET) who was developing a secondary MF (Table1).
RESULTS AND DISCUSSION
We observed a lower fixation of both tracers in the axial skeleton (AxS) and conversely a hyperfixation of 111In-Cl3 in the distal skeleton (DiS) and the spleen, this latter considered as pathognomonic of PMF or secondary MF (Figs1-2. Table1). The more advanced the process of marrow fibrosis and HSC externalization the more 111In-Cl3 splenic and DiS fixation. When associated to a low 111In-Cl3 fixation in the AxS it is suggestive of PMF. No other EMH localizations besides spleen were observed. Liver fixation interpretation was uneasy because both tracers show a significant fixation. Some questions remain unsolved as the mechanisms involved in the DiS recruitment in circumstances that this hematopoietic area was progressively inactive since birth and replaced with adipose tissue. Is DiS reactivation a consequence of HSCs that cannot seed in the physiological homing hematopoietic areas, which take part essentially in the AxS? Or is the fruit of HSC reactivation (and their microenvironment) already present in the DiS but simply dormant? In this latter case we do not know if HSCs carry the same molecular abnormalities of the malignant PMF clone.
Interestingly in the post ET MF patient, there was no 111In-Cl3 hyperfixation at the DiS. Conversely we observed a significant splenic fixation with both tracers and a low fixation with 99mTc in the AxS (Figs1-2). This evocates a sequence of hematopoietic reactivation starting in the spleen, followed by DiS recruitment and associated to a progressive hematopoietic regression in the AxS. HiM using these 2 radionuclides and SPECT/CT is a non-invasive procedure allowing larger and more precise visualization of the active/inactive hematopoietic areas in PMF. Moreover, regarding deep regions or high-weight patients, attenuation correction can show more uptake focus, potentially hidden because of soft-tissue interposition between emitting source and detector. This can represent an alternative to BM biopsies that also have the inconvenient to be limited in their investigation to the crista iliac. They may reflect the AxS but do not give any information neither on DiS nor on liver and spleen hematopoiesis.
CONCLUSIONS
The use of HIm allowed a good assessment of the extent and intensity of PMF hematopoiesis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal