Abstract
BACKGROUND: Ruxolitinib (RUX) is a potent JAK1/JAK2 inhibitor that has demonstrated superiority over standard therapies in the treatment of MF. In the phase 3 COMFORT studies, patients (pts) receiving RUX had improvements in splenomegaly and symptoms as well as longer survival compared with pts in the control arms. Study 258 extended these findings to pts with a platelet (PLT) count of 50 to ˂ 100 × 109/L (pts excluded from the COMFORT studies) and showed that starting at 5 mg bid with dose escalation to ≥ 10 mg bid offered similar benefits (Talpaz 2013). EXPAND aims to evaluate the safety of RUX and establish a maximum safe starting dose (MSSD) in pts with low PLTs.
METHODS: EXPAND is a phase 1b, dose-finding study (NCT01317875) in pts with MF and baseline PLTs 50 to 99 × 109/L. The study has 2 phases: dose escalation and safety expansion. In the dose-escalation phase, RUX doses were 5 mg bid, 5 mg AM/10 mg PM, 10 mg bid, 10 mg AM/15 mg PM, and 15 mg bid. A Bayesian logistic regression model with overdose control was used to guide dose escalation; intra-pt dose modification was allowed up to the MSSD in each stratum. Pts were assigned to 2 strata based on their baseline PLTs: S1, 75 to 99 × 109/L; S2, 50 to 74 × 109/L; doses in S2 were open if that dose and the next were deemed safe in S1. At data cutoff (20 Jan 2015), enrollment was ongoing in the safety expansion phase at the MSSDs; this interim analysis was preplanned for when the last patient enrolled in the dose escalation phase completed the core study (day 168).
RESULTS: 46 pts (S1, n = 27; S2, n = 19) received RUX; 12 pts were treated at each of the MSSDs for S1 (15 mg bid) and S2 (10 mg bid). Baseline pt characteristics were generally balanced across strata in all pts: 65% were aged ≥ 65 y, 48% were male, 74% had PMF (20% PPV-MF, 7% PET-MF), and 54% had high-risk MF. Median (range) baseline spleen length was 16 (5-30) and 12 (4-33) cm in S1 and S2, and median (range) hemoglobin was 104.7 (83-138) g/L in S1 and 100.0 (58-133) g/L in S2. Overall, 10 (37%) and 11 (58%) pts were still receiving treatment in S1 and S2. The primary reason for discontinuation was AEs (S1, 22%; S2, 21%). At the MSSDs, 1 pt (8.3%) in each group ended treatment due to AEs; 67% and 75% of pts at MSSDs in S1 and S2 were still on treatment at data cutoff.
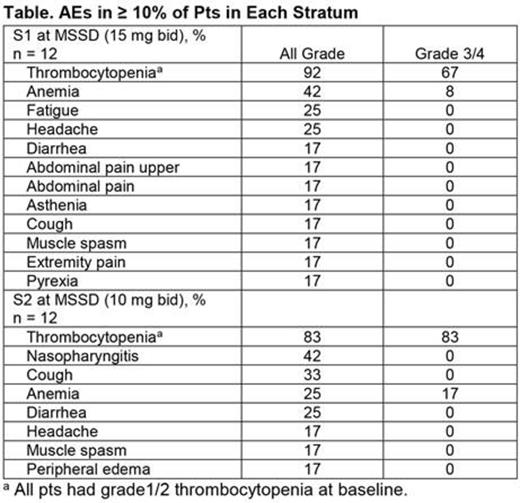
There were no DLTs in S1 and 1 DLT in S2 at 10 mg bid (grade 4 thrombocytopenia). AEs were consistent with the known safety profile of RUX. At the MSSDs, thrombocytopenia was the most common reason for dose modifications (S1, n = 8; S2, n = 8) but led to discontinuation for only 1 pt (S2). Deaths during the study included 1 cardiac arrest (S1), 1 sudden death (S1), 1 multi-organ failure (S2), and 1 transformation to AML (S2; ˃ 30 days after discontinuation), none of which were assessed as related to study drug.
The most common AEs overall (S1, S2) were thrombocytopenia (all grade: 82%, 79%; grade 3/4: 63%, 79%) and anemia (all grade: 56%, 37%; grade 3/4: 33%, 26%). Other common AEs (> 30% in either stratum) included diarrhea (33%) in S1, and cough (42%), nasopharyngitis (32%), and diarrhea (32%) in S2. At the MSSDs, the most frequent AE was also thrombocytopenia (Table).
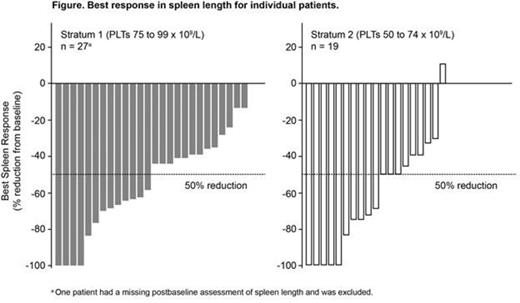
A ≥ 50% reduction in spleen length (spleen response) was achieved at week 24 in 41% of pts (7/17) in S1 and 30% of pts (3/10) in S2; 50% (13/26) in S1 and 68% (13/19) in S2 achieved a response at any time (Figure). At the MSSDs, 37.5% (3/8) and 40% (2/5) of pts in S1 and S2 achieved a spleen response at week 24; 50% (6/12) and 67% (8/12) of pts achieved a response at any time.
CONCLUSIONS: RUX was tolerated at starting doses of up to 10 or 15 mg bid in pts with MF and low PLTs (PLTs 50 to 74 × 109/L and 75 to 99 × 109/L). AEs were consistent with the known safety profile of RUX with no new or unexpected adverse findings. Spleen length reductions were observed across all dose levels, including the MSSDs, and are similar to those observed in pts without low PLTs. The study is currently open for enrollment and aims to evaluate the benefit vs risk of RUX further in this subgroup of pts, with emphasis on optimal dosing and long-term safety.
Vannucchi:Novartis: Other: Research Funding paid to institution (University of Florence), Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Shire: Speakers Bureau; Baxalta: Membership on an entity's Board of Directors or advisory committees. Gisslinger:Janssen Cilag: Honoraria, Speakers Bureau; Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Geron: Consultancy; Novartis: Honoraria, Research Funding, Speakers Bureau; AOP ORPHAN: Consultancy, Honoraria, Research Funding, Speakers Bureau; Sanofi Aventis: Consultancy. Harrison:Sanofi: Honoraria, Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau; CTI Biopharma: Consultancy, Honoraria, Speakers Bureau; Gilead: Honoraria; Shire: Speakers Bureau. Al-Ali:Celgene: Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Kiladjian:Novartis: Other: Travel grant; Research Funding paid to institution (Hôpital Saint-Louis et Université Paris Diderot); Novartis: Consultancy; Incyte Corporation: Consultancy. Nicolini:Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Ariad Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Mo:Novartis Pharmaceuticals Corporation: Employment. Atienza:Novartis Pharmaceuticals Corporation: Employment. Gopalakrishna:Novartis Pharma AG: Employment. te Boekhorst:CTI Biopharma: Consultancy; Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal