Abstract
Background: For pts with CML-CP treated with frontline IM, achievement of a sustained deep MR is one of the major criteria associated with successful treatment-free remission (TFR); other factors, including long duration of IM therapy and favorable Sokal risk score, have also been shown to be important. Factors affecting successful TFR in pts treated with frontline NIL are under investigation. Ongoing studies are evaluating TFR in pts who have received different durations of TKI treatment and achieved different durations of sustained MR4 (BCR-ABL1 ≤ 0.01% on the International Scale [BCR-ABL1IS ]) or MR4.5 (BCR-ABL1IS ≤ 0.0032%). Here, 6-y data from the ENESTnd trial of frontline NIL vs IM were analyzed to evaluate rates of MR4 and MR4.5 with each agent and estimate pts' rates of sustained response for ≥ 1 y while on NIL or IM treatment.
Methods: ENESTnd (NCT00471497) is an ongoing, randomized trial of NIL 300 mg twice daily (BID; n = 282) or NIL 400 mg BID (n = 281) vs IM 400 mg once daily (QD; n = 283) in pts with newly diagnosed CML-CP. Data from ENESTnd based on a minimum follow-up of 6 y for pts remaining on treatment were analyzed. Rates of deep MR were reported as cumulative incidence, with pts who achieved a response at or before each time point considered responders by that time point. Rates of sustained MR4 and MR4.5 on treatment among pts who achieved each response were estimated in each arm using a time-to-event analysis. In an exploratory analysis, the MR and treatment duration criteria for entering and attempting TFR in the ENESTfreedom trial (NCT01784068; a single-arm trial of pts with CML-CP who received ≥ 2 y of frontline NIL and achieved MR4.5 prior to enrollment) were applied a posteriori to determine the proportion of pts in each NIL arm of ENESTnd who were potential candidates for TFR, per the ENESTfreedom design (in the ENESTfreedom treatment consolidation phase, pts must maintain deep MR for 1 y [with real-time quantitative polymerase chain reaction assessments every 12 weeks, and with no assessment above MR4, ≤ 2 assessments between MR4 and MR4.5, and MR4.5 in the last assessment] on NIL 300 mg BID prior to attempting TFR).
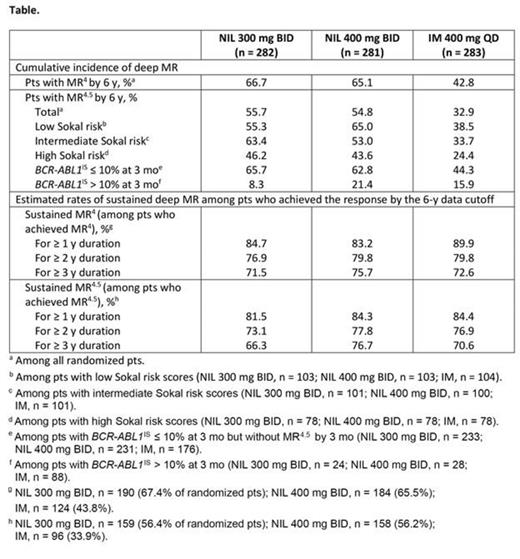
Results: With a minimum follow-up of 6 y in ENESTnd, 151 (53.5%), 155 (55.2%), and 127 (44.9%) pts in the NIL 300 mg BID, NIL 400 mg BID, and IM arms, respectively, remained on study treatment; median time on treatment was 5.8, 5.9, and 5.3 y, respectively. Cumulative rates of MR4 and MR4.5 by 6 y were higher with NIL vs IM; more pts achieved MR4.5 with NIL vs IM in all Sokal risk groups (Table). Among evaluable pts at 3 mo, more achieved BCR-ABL1IS ≤ 10% at 3 mo with NIL vs IM (NIL 300 mg BID, 234/258 [90.7%]; NIL 400 mg BID, 232/260 [89.2%]; IM, 176/264 [66.7%]); within each arm, rates of MR4.5 by 6 y were higher among pts who achieved BCR-ABL1IS ≤ 10% at 3 mo vs those with BCR-ABL1IS > 10% at 3 mo. Estimated rates of sustained MR4 and MR4.5 for ≥ 1, ≥ 2, and ≥ 3 y in pts achieving each response were high in all 3 arms; estimated rates of sustained MR4.5 for ≥ 1 y were 81.5%, 84.3%, and 84.4% in the NIL 300 mg BID, NIL 400 mg BID, and IM arms, respectively. By the data cutoff, 54.3% (153/282) and 54.8% (154/281) of pts in the NIL 300 mg BID and NIL 400 mg BID arms, respectively, had received ≥ 2 y of NIL treatment and achieved MR4.5 (the treatment duration and MR criteria for entering the ENESTfreedom treatment consolidation phase). In the 2 NIL arms, 37.9% (107/282) and 34.2% (96/281) of pts, respectively, had received ≥ 3 y of NIL treatment with sustained MRD for ≥ 1 y (the criteria for attempting TFR in ENESTfreedom).
Conclusion: In ENESTnd, NIL resulted in higher rates of MR4 and MR4.5 than IM. Once achieved, these deep MRs were durable in all 3 treatment arms, with > 80% of pts who achieved MR4.5 maintaining this response for ≥ 1 y. The higher rates of deep MR achieved with frontline NIL vs IM may enable more pts to qualify to attempt experimental TFR in a clinical trial. After a minimum follow-up of 6 y, 37.9% of pts (107/282) treated with NIL 300 mg BID in ENESTnd had the treatment duration and sustained deep MR equivalent to those required for attempting TFR in ENESTfreedom.
Hochhaus:Bristol-Myers Squibb: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; ARIAD: Honoraria, Research Funding. Saglio:Novartis: Consultancy, Honoraria; Ariad: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria. Hughes:Bristol-Myers Squibb: Honoraria, Research Funding; ARIAD: Honoraria, Research Funding; Novartis: Honoraria, Research Funding. Larson:Bristol-Myers Squibb: Consultancy; Ariad: Consultancy, Research Funding; Pfizer: Consultancy; Novartis: Consultancy, Research Funding. Taningco:Novartis Pharmaceuticals: Employment. Deng:Novartis Pharmaceuticals: Employment. Menssen:Novartis Pharma Basel Switzerland: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal