Abstract
Introduction: Follicular lymphoma grade 3 is recognized as a distinct entity in the World Health Organization classification of lymphoma. It is further classified into grade 3a and 3b depending on percentage of centroblasts. There is no consensus about its clinical course because some studies indicate an indolent behavior but others describe a more aggressive. Large systematic studies are missing in particular for 3b follicular lymphoma which is often considered as a separate entity.
Methods: We performed a retrospective multicentric study on a group of 3b FL patients diagnosed in nine Italian FIL centers between November 2002 and January 2015. Planned inclusion criteria at enrollment were first line Rituximab containing regimen treatment and diagnostic samples availability for central pathologic review. Aim of the study was to determine clinical response, OS and PFS. Tumor response was based on the International Working Group response criteria. Survival analysis was performed with Kaplan-Meier method.
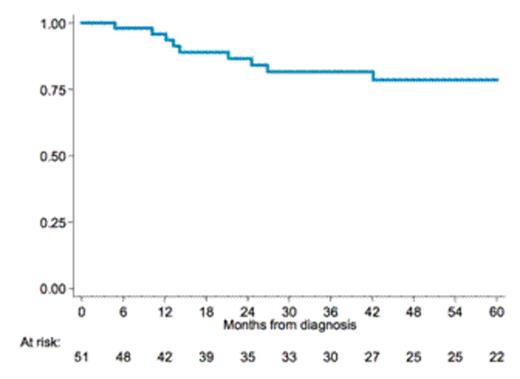
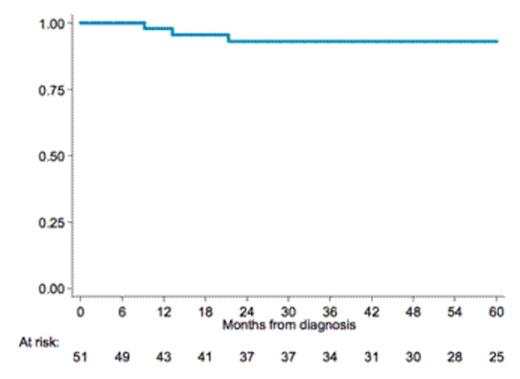
Results: We enrolled a total of 51 patients, 50 evaluable for response at the time of analysis; median age was 62 yrs (range 48-71), 29 (56%) in stage III-IV, 10 (20%) with B symptoms. First line treatment was R-CHOP in the majority of patients 47 (92%), R-Bendamustine and R-CVP in 2 (4%) respectively. Seven patients (14%) received Rituximab maintenance after first line, six (12%) underwent high dose chemotherapy and autologous stem cell transplant (ASCT) as consolidation therapy and 5 (10%) were treated with local radiotherapy on residual disease. We observed CR in 48 patients (96%), PR in 1 (2%), PD in 1(2%). Ten patients relapsed or progressed after first line treatment and four of them died, three for progressive disease and one due to senile dementia while in CR. No relapses were recorded in pts receiving Rituximab maintenance but the advantage was not statistically significant and the number of patients receiving maintenance was low. With a median follow up of 63 months from diagnosis (IQR 33-82), 3-yrs PFS and OS rates were 82% and 93% (fig 1 and 2) with the evidence of a plateau in both survival curves after 5 years observation. Central pathologic review is ongoing.
Conclusion: With the limit of a retrospective analysis our study confirms the clinical benefit of a combined modality treatment with Rituximab plus antracycline-containing chemotherapy in patients with 3b FL. Our results compare favorably with those previously reported in studies without Rituximab, that failed to show a plateau with 3-yrs PFS ranging between 22% and 52%. This results need to be confirmed with a longer follow up and after the planned pathologic review.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal