Abstract
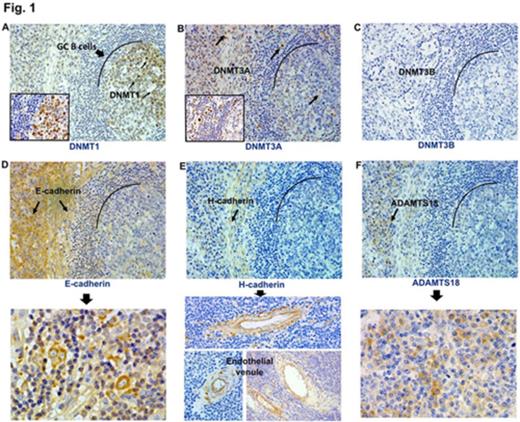
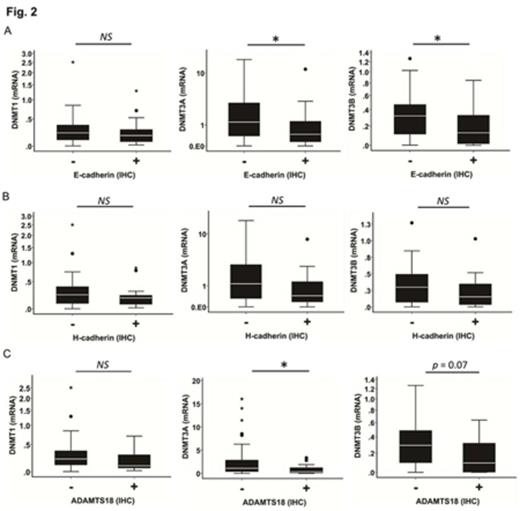
In situ, patterns of expression of DNMTs (DNA methytransferases) in normal reactive tonsillar tissue have been examined. Difference in pattering of expression of DNMTs and TSG (Tumor suppressor genes) proteins in lymphoid tissue section is an important question in relation to their association with each other as well as relationship to mRNA gene expression level. In order to examine this issue, we examined DNMTs and TSG proteins expression by immunohistochemistry in sections of paraffin-embedded specimens obtained from 33 subjects of lymphoma and 16 subjects of Non-malignant tissues after receiving written informed consent. The specimens were stained with anti-DNMTs (DNMT1, DNMT3A and DNMT3B) and anti-TSG (E-cadherin, H-cadherin and ADAMTS18) antibodies. In addition, using fresh-frozen optimal cutting temperature (OCT) compound-embedded tissue specimens before any treatment, we examined mRNA expression levels and promoter methylation status of E-cadherin (CDH1), H-cadherin (CDH13) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS18) using quantitative real-time PCR (qRT-PCR) and methylation-specific PCR (MSP), respectively. The expression of nuclear DNMTs proteins (DNMT1 and 3A) in lymphoma section was observed in [17/33 (51%); 27/33 (81%)], whereas in non-malignant tissues was [14/16 (87.5%); 13/16 (81%)], respectively. The DNMT3B protein expression was not detected in our tissue samples, which might be explained by the fact that DNMT3B characterized by alternative splicing as shown previously. Membrane proteins (E-cadherin, H-cadherin and ADAMTS18) showed low expression [12/33 (36%); 10/33 (30%); 6/33 (18%), respectively], when compared to non-malignant tissue sections [12/16 (75%); 7/16 (43%); 8/16 (50%), respectively]. The expression levels of CDH1, CDH13 and ADAMTS18 mRNAs were non-significantly reduced in their corresponding protein negative expression compared to the levels in cases with positive protein expression (p =0.112, p =0.378, p =0.077, respectively). We could not find any correlation between mRNA/protein expression levels of DNMTs and the methylation status of CDH1, CDH13 and ADAMTS18. Importantly, by immunostaining especially in non-malignant lymphoid tissues, we found that DNMT1 was highly detected in germinal center B cells (GC B cells) with gradual decrease or no expression in the mantle, marginal, interfollicular and T cells zones. Whereas DNMT3A was preferentially and scattered like expressed in the cells of the surrounding zones out of the germinal centers. Furthermore, E-cadherin, H-cadherin and ADAMTS18 proteins expression were detected on the cell surface membrane of the cells outside the GC but at rates somehow more than those cells inside the GC (Fig. 1). This is supported by the significant association observed between the frequency of DNMT3A with both E-cadherin and ADAMTS18, protein expressions (Chi square: p <0.05), while no association with H-cadherin protein expression. In addition, DNMT1 protein expression did not show significant association with the protein expressions of E-cadherin, H-cadherin and ADAMTS18. Moreover, the mRNA expression levels of DNMT3A and 3B showed high significant levels (p <0.05) in cases with negative protein expressions of both E-cadherin and ADAMTS18 when compared to cases with positive protein expressions (Fig. 2A and C). The DNMT1 mRNA expression level did not show any significant difference between the negative and positive protein expressions of E-cadherin, H-cadherin and ADAMTS18 (Fig. 2B). Furthermore, there was no significant association between the mRNA levels of DNMTs and H-cadherin protein expression. Expression of H-cadherin protein was frequently observed in the endothelial venules and trabeculae of the lymphoid tissues (Fig. 1) which might cause of its lack of association with both DNMT1 and DNMT3A. In conclusion, these results indicate that as a result of differences in pattering of DNMTs and TSG protein expressions detected in lymphoid tissues by immunohistochemistry staining, it might be one of the reasons of the association with each other and their mRNA expression levels across the spectrum of lymphomas and non-malignant lymphoid tissues.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal