Abstract
Background: Acute myeloid leukemia (AML) is an aggressive hematological malignancy which has an incidence of 40 children on average per year in the Netherlands and Belgium combined (population of 28 million). Despite the fact that almost all children achieve complete remission (CR), 30-40% of patients eventually relapse. Since survival after relapse is poor (35-40%), the overall survival (OS) of pediatric AML remains relatively low (~70%) compared to the increasing remission rates. The most effective strategy to improve the dismal outcome of AML patients is thus to prevent relapse.
Over the last decades accumulating evidence is suggesting that AML develops in a hierarchical structure; originating from hematopoietic stem cells which are transformed to leukemia initiating cells, also referred to as leukemic stem cells (LSC). LSC possess self-renewal capacity and are more resistant to therapy. Therefore, LSC are supposed to be responsible for outgrowth of both the initial leukemia and the relapse. This holds true for adult AML, where the frequency of LSC at diagnosis has shown to be of importance for clinical outcome. However, while it has been shown that a high number of immature cells (CD34+/CD38-/CD45-/low) associates with an increased risk of relapse in pediatric AML, relatively little is known about the prognostic impact of LSC within this compartment.
RAEB-t. Flow-cytometric LSC characterization was performed on either bone marrow (BM) or peripheral blood (PB) from AML patients collected at diagnosis. Purified white blood cells (WBC) were obtained after lysing the red blood cells using lysing solution (Pharm Lyse or FACSLysing, BD Biosciences) and were subsequently incubated with monoclonal antibody combinations and analyzed using an 8-color flow cytometer approach. LSC were defined as CD34+ CD38- cells with aberrant expression of CD123, CD7, CD56 or CD2.
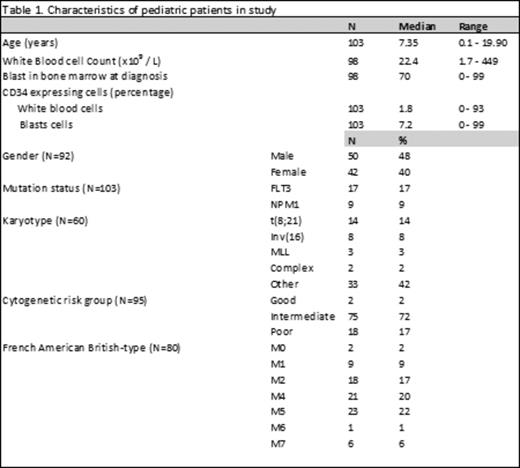
Results: Data were available from 103 patients and patient characteristics are listed in Table 1. In this cohort relapse-free survival (RFS) was 59.4%. Fortunately 56.4% of relapsed patients achieved a second CR and OS was 85.4%. LSC-load is defined as % of aberrant CD34+ CD38- cells within the CD34+ compartment. In our pediatric cohort, 17 patients (16.5%) had no expression of CD34 on blast cells at all (CD34null) and consequently no aberrant CD34+ CD38- LSC could be detected. Absence of CD34 has often been associated with good prognosis in literature. In our cohort relapse-rate seemed to follow this trend (4 out of the 17 CD34null patients (23.5%) vs. 34 out of the 83 CD34positive patients (41%)) but did not differ significantly (Plogrank = 0.196).
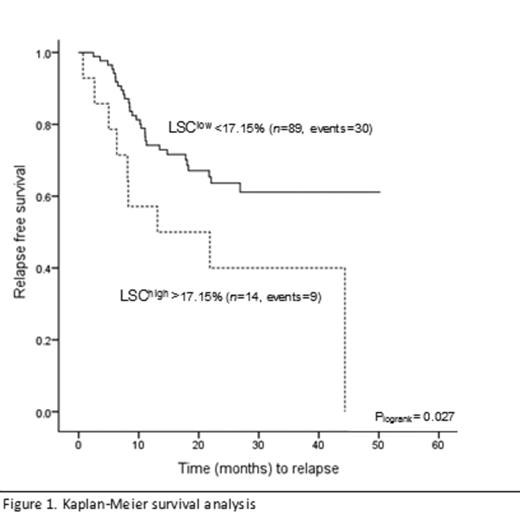
ROC curve analysis showed that a cut-off of 17.2% LSC at diagnosis was associated with the occurrence of developing relapse with a specificity of 92%. Kaplan-Meier survival analysis, as depicted in Figure 1, showed a significant association between a high LSC-load and impaired RFS (34% relapses in LSClow vs. 64% relapses in LSChigh) (Plogrank= 0.027). Univariate analysis showed that next to LSC percentage, FLT3 mutation status and WBC count were significantly associated with RFS. After multivariate adjustment (taking into account cytogenetic risk groups and mutational status) LSC frequency was the only independent predictor of relapse or RFS (HR 2.3, 95% CI 1.1-4.8, p=0.032).
Conclusion: Our results confirm data from adults and reveal that the frequency of LSC at diagnosis in pediatric AML patients can distinguish patients more likely to fail current treatment regimens as these patients develop significantly more relapses). Identifying this patient group creates opportunities for more personalized medicine and the development of therapies directed against LSC, sparing normal hematopoietic stem cells.
Kaspers:Janssen-Cilag: Research Funding. Cloos:Takeda: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal