Abstract
Background:
Recent genomic studies have identified somatic mutations in leukemia genes in asymptomatic individuals without known hematologic disease. These mutations lead to clonal expansion of hematopoietic cells, in the absence of clinically overt hematologic transformation. This entity is thought to be analogous to other precursor conditions such as monoclonal gammopathy of undetermined significance (MGUS) and monoclonal B-cell lymphocytosis (MBL); consistent with this notion, a subset of patients with clonal hematopoiesis can subsequently develop myeloid malignancies, including myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). However, the incidence of clonal hematopoiesis in patients with solid tumors has not been extensively studied. Moreover, most genomic profiling platforms used to identify somatic mutations in epithelial tumors use blood as the matched normal control, which may lead to the identification of clonal hematopoiesis in a subset of cancer patients. We therefore sought to assess for clonal hematopoiesis in patients with solid tumors seen at Memorial Sloan Kettering Cancer Center (MSKCC) who were profiled using paired tumor/blood sequencing which is primarily designed to identify tumor-specific mutations.
Methods:
The study population included patients at MSKCC who were consented on protocol NCT01775072, “Tumor genomic profiling in patients evaluated for targeted cancer therapy.” All patients had tumor and blood genomic profiling sequenced using the MSK-IMPACT hybridization capture-based next-generation sequencing assay, which encompasses all protein-coding exons of 410 cancer-associated genes. For assessment of the incidence and distribution of blood mutations, 3,964 patients consented were evaluated. Patients with known active hematologic malignancies at time of sequencing were excluded from analyses. For comparisons regarding clinical correlations, 2,146 patients were included for whom detailed chart review has been performed.
We investigated for the presence of “hotspot” mutations, collected from COSMIC database (v71), in matched blood DNA. Mutations were scored as present in the normal blood if they were detected in more than 8 reads with a variant allele frequency (VAF) greater than 2% which was at least twice the VAF seen in tumor. For cases where at least one mutation exceeded these thresholds, we reduced the VAF threshold in blood to 1% to detect secondary events. Mutations where VAF in the normal blood and the tumor sample were both greater than 30% were excluded as likely germline events.
Results:
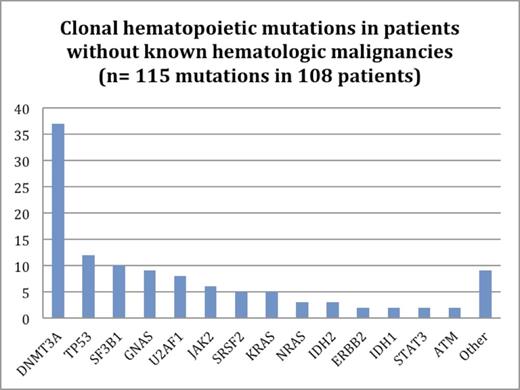
Clonal hematopoiesis was identified in 108/3,964 solid tumor patients who had paired tumor/blood sequencing (2.7% of all patients); 7 patients had 2 unique mutations. DNMT3A mutations were most frequent, occurring in 34% of patients with clonal hematopoiesis (Figure 1). 107/115 mutations (93%) were in known leukemia-associated genes.
We collected and analyzed detailed clinical parameters for 2,146 patients, including 42 patients (2.0%) with clonal hematopoiesis. The mean age at the time of testing was 62.1 years in patients with clonal hematopoiesis and 57.2 years in patients without evidence of clonal hematopoiesis (p = 0.015). When comparing baseline blood parameters, there were no statistically significant differences between the two groups. 62% of patients with clonal hematopoiesis had previous radiation therapy compared to 45% of patients without clonal mutations in their blood (p = 0.046). There was no statistically significant difference in the proportion of patients who had received previous chemotherapy (71% of patients in each group).
On prospective follow up of patients with clonal hematopoiesis, no patients have progressed to develop overt MDS or AML, though median follow up was limited (median 11.7 months, range 5.5-16.7 months).
Conclusions:
Clonal hematopoiesis in solid tumor patients without known hematologic disease is common and is associated with increasing age and prior radiation therapy. In our cohort, no patients with clonal hematopoiesis progressed to overt MDS or AML though follow up is limited. Ongoing prospective observation of these patients is imperative to determine the clinical impact of these mutations after ongoing exposure to cytotoxic therapy. Further data including updated clinical and genomic correlates will be presented.
Reference:
Cheng DT et al. J Mol Diagn 2015 May; 17(3): 251-64.
Hyman:Chugai Pharma: Consultancy; Biotherapeutics: Consultancy; Atara: Consultancy, Honoraria. Levine:Loxo Oncology: Membership on an entity's Board of Directors or advisory committees; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees; Foundation Medicine: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal