Abstract
Background: The Joint Outcome Study (JOS), a randomized controlled trial of prophylactic vs episodic factor VIII for treatment of severe hemophilia A, conclusively showed that children started on prophylactic factor VIII before age 30 months had better joint outcomes by age 6, as measured by MRI, joint physical exam scores, and annual number of joint bleeds(Manco-Johnson, et al, NEJM 2007). However, variation in individual outcomes existed within each treatment group that was not well-explained. We measured hemostatic and fibrinolytic factors in plasma samples from the Joint Outcome Study to determine other contributors to bleeding outcomes.
Methods: Samples from 65 patients in the JOS were evaluated. Factors assayed in the University of Colorado Hemophilia & Thrombosis Treatment Center Research Laboratory included: average factor VIII (FVIII) trough (prophylaxis) or baseline (episodic) activity levels; Von Willebrand Factor antigen (VWF); tissue factor pathway inhibitor (TFPI); thrombin activatable fibrinolysis inhibitor (TAFI); factor V Leiden measured by activated protein C resistance (APC-R) assay; thrombin-antithrombin complexes (TAT); and euglobulin lysis time (ELT). Results were correlated with outcomes at age 6 years, including: number of joint bleeds, total number of bleeds, MRI score, and joint physical exam score. Joint failure on MRI was defined as an MRI score of 7-10 out of 10 possible points on any joint. Linear regression was used to compare each variable with each outcome. Prophylactic and episodic groups were analyzed separately and together.
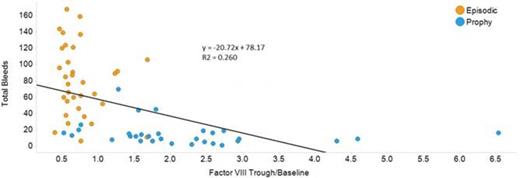
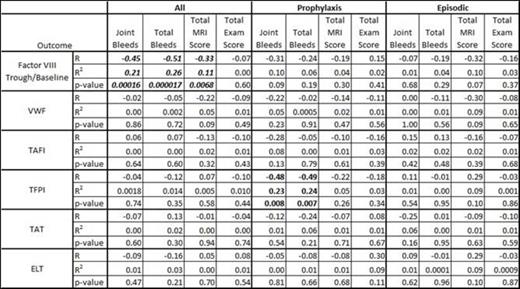
Results: When both groups were evaluated together, linear regression showed significant inverse correlations between FVIII trough/baseline levels and either joint bleeds (Figure 1, p < 0.001), total bleeds (p < 0.0001), or total MRI score (p < 0.01), although episodic patients had significantly more bleeds and higher MRI scores than prophylaxis patients. This relationship was not seen if prophylaxis and episodic groups were evaluated separately (Table 1). Specifically, boys in the prophylaxis group showed no significant relationship between trough and bleeding or MRI outcomes when analyzed separately, likely because the rate of bleeds on prophylaxis was so low at any trough level. No significant association between factor VIII trough/baseline levels and physical exam joint scores was found. When prophylaxis and episodic groups were evaluated separately, the only significant association between any outcome and any coagulation factor was TFPI, which unexpectedly had an inverse relationship with joint and total bleeds (Table 1). When results were stratified by joint preservation vs. joint failure on MRI, subjects with joint failure had slightly lower FVIII trough/baseline, VWF, and TAFI levels, but these results were not significant. Two children were found to have coincidental Von Willebrand disease (VWF antigen levels of 18% (episodic arm), 31% (prophylactic arm)); the child on episodic treatment suffered joint failure on MRI. Three children (2 in episodic arm and 1 in prophylaxis arm) had factor V Leiden heterozygosity, and none of these children had joint failure.
Conclusion: In the closely monitored and extensively studied participants of the JOS, higher FVIII trough/baseline levels were associated with decreased bleeding rates. In contrast, physiologic variation in constitutional levels of VWF, TAFI, and TAT, while modestly and non-significantly lower in children with joint failure on MRI, did not affect bleeding rates. While it is tempting to attribute bleeding to coincidental VWD and joint preservation to factor V Leiden heterozygosity, the data are too limited for conclusions. Use of factor VIII prophylaxis remains the most powerful predictor of joint outcome in children with severe hemophilia A.
Linear regression statistics comparing coagulation proteins to each outcome.
Shapiro:Baxalta, Novo Nordisk, Biogen,: Membership on an entity's Board of Directors or advisory committees; Bayer Healthcare, Baxalta, Biogen, CSL Behring, Daiichi Sankyo, Kedrion Biopharma, Octapharma, OPKO, ProMetic Life Sciences, PTC Therapeutics, and Selexys: Research Funding; Biogen: Speakers Bureau; Baxalta, Novo Nordisk, Biogen, ProMetic Life Sciences, and Kedrion Biopharma: Consultancy. Recht:Baxalta: Research Funding; Novo Nordisk: Research Funding; Biogen: Research Funding; Kedrion: Membership on an entity's Board of Directors or advisory committees. Brown:Baxalta: Research Funding. Leissinger:Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Baxter: Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL Behring: Membership on an entity's Board of Directors or advisory committees, Research Funding; Biogen: Research Funding; Kedrion: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees. Kempton:Biogen: Consultancy; Novo Nordisk: Consultancy; Baxalta: Consultancy. Lane:Bayer Pharmaceuticals: Consultancy; Baxter Healthcare: Consultancy. Manco-Johnson:CSL Behring: Honoraria; Baxalta: Honoraria; Bayer Healthcare: Honoraria, Research Funding; BiogenIdec: Honoraria; Novo Nordisk: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal