Abstract
Immune Thrombocytopenia (ITP) is an autoimmune disorder characterized by isolated low platelet count (<100x109/L) with a variable risk of bleeding. The prevalence of ITP in France is 25/100000. While drug therapy is indicated in patients with platelet count less than 30x109/L and or with bleeding symptoms, discussions remain about therapeutic strategy to be implemented. The SATURNE study aimed to describe the current therapeutic management of adult ITP patients, to focus on TPO-RAs treated patients, and to assess changes in real life treatment strategy since TPO-RAs' approval.
This study was carried out in referral and non-referral centersby hematologists and internists from hospitals or clinics in France. Enrolled patients were adults suffering from persistent (3 to 12 months after diagnosis) or chronic (>12 months) ITP. Patients with a newly diagnosed ITP (<3 months) and patients with secondary ITP (viral infection, lupus, etc.) were excluded.
Data were collected online by investigators through an eCRF at inclusion (M0). A subgroup of patients initiating a TPO-RAs treatment during the study period was followed up at M3, M6, M12, M18 and M24. Only M0 data are presented in this abstract as interim analysis.
Overall, 48 investigators included 333 patients (278 with chronic ITP and 55 with persistent ITP) over a 19 months period (2012 to 2013). Figure 1 displays the main characteristics including comorbidities and laboratory tests performed at diagnosis. Half ofthe patients (53%) had bleeding manifestations at ITP diagnosis; 10% at time of inclusion. ITP was mainly diagnosed by a hematologist (53%) or an internist (32%) and less frequently by a general practitioner (11%).
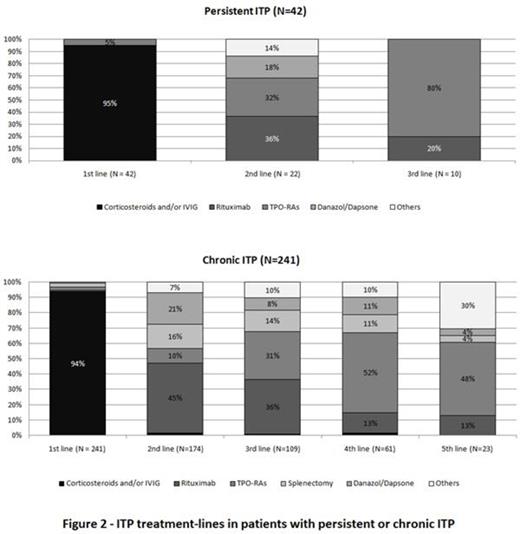
Patients completed a median of 2 treatment lines before entering the study. Figure 2 shows treatment-lines distribution according to the ITP phase. Most patients had been treated with corticosteroids ± intravenous immunoglobulin (IVIG) (83%) as 1st line treatment. Rituximab was the preferred 2nd line option, far prior to splenectomy (44% vs 14%).
A total of 144 patients (123 chronic/ 21 persistent ITP) received TPO-RAs (39% romiplostim/ 33% eltrombopag / 15% both / 13% non specified): 6%, 20%, 34%, 28% and 12% respectively as a 1st line treatment, 2nd, 3rd, 4th, 5th and beyond. At inclusion 75% were still on TPO-RAs. Recently diagnosed patients received 2nd line TPO-RAs in higher proportions: 40% (of 46 patients diagnosed <2 years) vs 15% (of 65 patients diagnosed 2-5 yrs ago) and 7% (of 72 patients diagnosed >5 yrs). TPO-RAs became the 3rd line most used treatment (over 76% for diag. <2 yrs). In parallel, the use of splenectomy decreased from 31% (diag. >5 yrs) to 9% (<2 yrs) in 2nd line, and from 16% to 5% in 3rd line. TPO-RAs treated patients had a more severe ITP, in particular at diagnosis. More patients in this group showed platelet counts less than 30.109/L (71% vs 51%, p<0.0001 at diagnosis / 23% vs 10%, p<0.001 at inclusion), and bleeding manifestations (64% vs 44%, p<0.001 at diagnosis/12% vs 8%, p=0.24 at inclusion). They received an average of 3.2 lines of treatment (against 1.7 in TPO-RAs' non-treated patients, p<0.0001).
The SATURNE study supports epidemiological trends observed in current practice in terms of patients and ITP characteristics, and provides current data on comorbidities.
The results highlight the increasing use of TPO-RAs as 2nd and 3rd lines for ITP treatment and the decrease of splenectomy use over time. Initiated in 2012, respectively 1 and 2 years after eltrombopag and romiplostim approval, the SATURNE study points out the changes of the management of adult ITP in France.
| . | N=333 . |
|---|---|
| Age | 57 ± 20yr |
| Women | 190 (57%) |
| Main ITP characteristics | |
| Chronic/Persistent ITP | 278 (84%)/55 (16%) |
| Mean ITP duration* | 6 ±8yr |
| Platelet count* - diagnosis - inclusion | 33±31.109/L 100±83.109/L |
| Hemorrhagic manifestations - diagnosis - baseline | 176 (53%) 32 (10%) |
| White blood cell* | 8±3.109/L |
| Hemoglobin* | 14±5g/dl |
| Globular volume* | 90±6fl |
| Comorbidities since ITP diagnosis | |
| At least once | 36% |
| Hypertension | 17% |
| Diabetes | 8% |
| Benign/malignant tumors | 8% |
| Cardiovascular disease | 6% |
| Diagnostic tests performed at ITP onset | |
| Viral serology tests | 96% |
| Blood smear | 93% |
| Blood coagulation | 93% |
| Marrow aspirate | 78% |
| Antiplatelet antibody | 52% |
| ITP treatment (at least once since diagnosis all lines combined) | |
| Corticosteroids and/or IVIG | 275 (83%) |
| Rituximab | 146 (44%) |
| TPO-RAs - eltrombopag - and/or romiplostim | 144 (43%) 69 (48%) 78 (54%) |
| Splenectomy | 59 (18%) |
| *mean ± SD | |
| . | N=333 . |
|---|---|
| Age | 57 ± 20yr |
| Women | 190 (57%) |
| Main ITP characteristics | |
| Chronic/Persistent ITP | 278 (84%)/55 (16%) |
| Mean ITP duration* | 6 ±8yr |
| Platelet count* - diagnosis - inclusion | 33±31.109/L 100±83.109/L |
| Hemorrhagic manifestations - diagnosis - baseline | 176 (53%) 32 (10%) |
| White blood cell* | 8±3.109/L |
| Hemoglobin* | 14±5g/dl |
| Globular volume* | 90±6fl |
| Comorbidities since ITP diagnosis | |
| At least once | 36% |
| Hypertension | 17% |
| Diabetes | 8% |
| Benign/malignant tumors | 8% |
| Cardiovascular disease | 6% |
| Diagnostic tests performed at ITP onset | |
| Viral serology tests | 96% |
| Blood smear | 93% |
| Blood coagulation | 93% |
| Marrow aspirate | 78% |
| Antiplatelet antibody | 52% |
| ITP treatment (at least once since diagnosis all lines combined) | |
| Corticosteroids and/or IVIG | 275 (83%) |
| Rituximab | 146 (44%) |
| TPO-RAs - eltrombopag - and/or romiplostim | 144 (43%) 69 (48%) 78 (54%) |
| Splenectomy | 59 (18%) |
| *mean ± SD | |
Michel:Roche: Research Funding; AMGEN: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GSK: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees. Adoue:GSK: Other: Symposium presentations; AMGEN: Other: Symposium presentations; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; OCTAPHARMA: Other: Symposium presentations; LFB: Other: Symposium presentations; PFIZER: Other: Symposium presentations; ACTELION: Other: Symposium presentations. Cheze:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees. Coppo:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees. Leclerc-Teffahi:Novartis: Employment. Fernandes:Novartis: Other: CRO. Texier:Novartis: Other: CRO.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal