Abstract
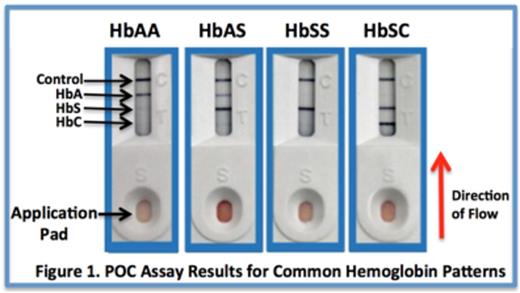
Sickle cell disease (SCD) is a common and life-threatening inherited disorder of hemoglobin, affecting over 400,000 newborns annually. The majority of these births occur in low-resource countries, particularly in sub-Saharan Africa, where limited access to accurate diagnostics results in early mortality. Accurate diagnosis of SCD currently relies upon analytical techniques that are relatively expensive and tedious, requiring equipment, electricity, and laboratory expertise. In Africa, outside of referral and regional hospitals, the availability of accurate SCD diagnostics is extremely scarce. A simple, rapid, accurate, and inexpensive point-of-care (POC) method for diagnosing SCD in limited resource settings would represent a tremendous advance for the management of SCD worldwide. We evaluated a novel point-of care SCD immunoassay (Sickle SCANTM, Biomedomics, Inc., Research Triangle Park, NC) that utilizes lateral flow technology and antibody-mediated detection of hemoglobin variants, with the goal of determining the accuracy, sensitivity, specificity, and ease of identifying the presence of hemoglobin A (HbA), hemoglobin S (HbS), and hemoglobin C (HbC) using blood samples from patients with a variety of hemoglobin patterns. Blood samples collected in EDTA were first tested by HPLC to determine the percentages of normal and abnormal hemoglobins and were then tested using Sickle SCAN. Mixtures of specific hemoglobin combinations were also created to determine the sensitivity of the POC assay for detecting low concentrations of HbA, HbS, and HbC. The Sickle SCAN kit includes tubes prefilled with 1.0mL of buffer, which lyses erythrocytes and releases hemoglobin. Whole blood samples were tested by adding 5µL of whole blood from the EDTA tube to the prefilled buffer container, mixing by inverting the tube three times, discarding 3 drops of the mixed solution and then applying 5 drops to the testingcartridge. Dried blood spot samples were also tested by dropping a 3mm punch into the prefilled buffer container, mixing, discarding 3 drops, and applying 5 drops to the testing cartridge. Five minutes after sample application, two independent and masked clinicians visually scored each sample for the presence/absence of each potential band (HbA, HbS, HbC, and Control). Figure 1 illustrates the visual results of common hemoglobin patterns.
A total of 50 samples were evaluated using the POC device, including 32 whole blood samples, 7 dried blood spots, and 11 samples artificially created to contain known low concentrations of HbA, HbS, and HbC. Temperature and stability were evaluated using 10 additional samples that were stored at 37C for up to 30 days. In order to identify potential interference by hemoglobin variants, samples included many different types of hemoglobin (HbA2, Hb Bart's, HbD, HbE, HbF, Hb Lepore, Hb Hope, Hb I-Texas, and Hb G-Philadelphia). From whole blood samples, HbA, HbS, and HbC were easily detected in both heterozygous and homozygous samples, but the intensity of individual bands did not correlate with actual percentages. Newborn samples with high fetal hemoglobin (HbF) were also easily and accurately analyzed for the presence of HbA, HbS, and HbC, with no obvious interference from HbF. The presence of common variant hemoglobins also did not cross-react, but both observers noted a faint HbA band for a newborn sample with a HbFE pattern. For samples artificially created to contain low concentrations of HbA, HbS, or HbC, these hemoglobin were detected at concentrations of <5%. Dried blood spot samples also yielded clear positive bands, without loss of sensitivity or specificity. Devices stored at 37C and blood samples stored at 4C for up to one month gave identical results to those stored at room temperature. These analyses indicate that the Sickle SCAN POC device was simple, robust, and highly sensitive and specific for detecting HbA, HbS, and HbC, even in very low percentages. The device easily and rapidly detected common hemoglobins, but was not quantitative. Specificity was excellent even in the presence of HbF and common variants, with the possible exception of HbE. The ability to obtain rapid and accurate results with both liquid blood and dried blood spots, including those with newborn high-HbF phenotypes, suggests that this device is suitable for large-scale screening of SCD in limited resource settings.
Ware:Eli Lilly: Other: DSMB membership; Biomedomics: Research Funding; Bayer Pharmaceuticals: Consultancy; Bristol Myers Squibb: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal