Abstract

Background: Induction therapy with daunorubicin (Dauno) & cytarabine (Ara-C) [DA] has been the standard of care for eligible older adults (age ≥ 60 years) with newly diagnosed acute myeloid leukemia (AML) for over 2 decades. Single agent Clofarabine (CLO) induction & consolidation (Consol.) therapy has demonstrated important clinical activity in this age group in large phase II studies. Lower induction mortality (IM) & similar reported complete remission rate (CR) & overall survival (OS), as well as notable activity in those with higher risk disease features [including unfavorable cytogenetics, therapy-related AML (t-AML) & prior antecedent hematologic disorder (AHD)] raises the possibility that a non-Ara-C-based regimen could achieve similar or superior OS with lower toxicity.
Methods: We performed a randomized United States Intergroup Phase III trial of single agent CLO [30mg/m2 x 5 days induction; 20 mg/m2 re-induction (if indicated) & 2 cycles Consol.] vs. standard DA therapy [Dauno 60mg/m2 D1-3 & Ara-C 100mg/m2 D1-7 induction x 1-2 cycles; 2 cycles Consol. with Ara-C (1.5g/m2 Q12hrs D1-6 age 60-69; once daily if age 70+)] in patients (pts) age ≥ 60 yrs with newly diagnosed AML. Patients with serum creatinine >1.0 (or GFR <60 mL/min) and those with AML-M3 and ECOG performance status >3 (PS>2 if age 70+ yrs) were excluded. Randomization was stratified by age (60-69 vs. 70+), t-AML, & AHD. Pts with HLA-matched donor were eligible for allogeneic transplantation (AlloHCT) after induction, and those completing Consol. were eligible for randomization #2 (R#2) to maintenance decitabine [20mg/m2 x 3D, monthly x 1 year] versus observation. With a target accrual of 747, E2906 was powered to determine non-inferiority [and possible superiority] of CLO vs. standard DA, and primary endpoint was OS. A weighted statistical analysis was performed to account for confounding impact of R#2. AlloHCT patients were censored at transplant in this analysis. Responses & cytogenetics were confirmed centrally and OS & CR rates were monitored by an independent Data Safety Monitoring Committee (DSMC) at pre-specified time points.
Results: As of Feb 23, 2015, 727 pts were randomized. Median age was 68 years (range 60-86); 57% were male, and 38% were age ≥70 yrs. Treatment arms are well balanced for all baselineclinical & AML characteristics, & 30% had unfavorable cytogenetics. Of 659 with complete treatment information reported, 30.4% on DA vs. 40.1% on CLO received 2 cycles of induction (p=0.006).
Median follow-up of surviving patients is 7.6 months.
shows early treatment results (CR, toxicity) for the 686 pts randomized as of Dec 23, 2014 (2 months prior to study end, & excluding 90 with ongoing response evaluation).
| . | DA . | CLO . | p-value . |
|---|---|---|---|
| CR/CRi | 43.8% | 42.8% | p=0.87 |
| 30-day mortality | 8.5% | 7.9% | p=0.89 |
| 60-day mortality | 14.9% | 13.1% | p=0.58 |
| Gr 4-5 Non-Heme Tox. Induction | 27% | 19% | p=0.02 |
| Gr 4-5 Non-Heme Tox. Consol. | 20% | 7% | p=0.001 |
| . | DA . | CLO . | p-value . |
|---|---|---|---|
| CR/CRi | 43.8% | 42.8% | p=0.87 |
| 30-day mortality | 8.5% | 7.9% | p=0.89 |
| 60-day mortality | 14.9% | 13.1% | p=0.58 |
| Gr 4-5 Non-Heme Tox. Induction | 27% | 19% | p=0.02 |
| Gr 4-5 Non-Heme Tox. Consol. | 20% | 7% | p=0.001 |
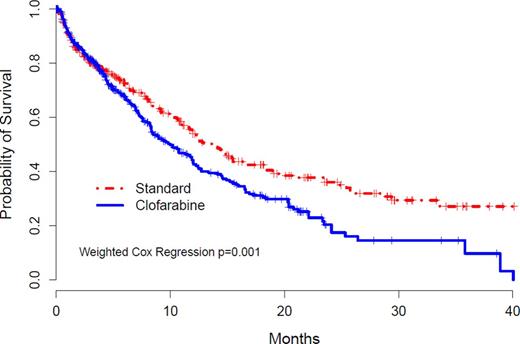
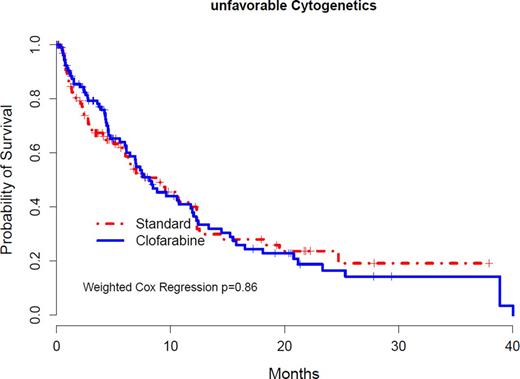
374 pts have died (174, DA; 200, CLO) & significantly inferior OS was observed for CLO vs. DA [Hazard Ratio (HR) 1.41 (95% CI 1.12-1.78)] (Fig. 1). Planned subgroup analyses were performed (Table 2) demonstrating significant differences in OS after CLO for patients age 60-69 yrs, without AHD, & with intermediate risk cytogenetics; but not for those with Unfav. Cytogen. (Fig. 2) or t-AML. Based on the primary weighted analysis, DSMC recommended suspension of new accrual to E2906 on Feb 23, 2015 & all active patients on CLO were transitioned to DA Arm.
| . | N . | HR CLO/Standard (95% CI) . |
|---|---|---|
| *Weighted OS | 727 | 1.41 (1.12-1.78) |
| Unweighted OS | 727 | 1.23 (1.00-1.50) |
| Age 60-69 | 449 | 1.48 (1.10-1.99) |
| Age 70+ | 278 | 1.34 (0.93-1.93) |
| Intermed. Risk Cytogen. | 378 | 1.77 (1.27-2.47) |
| Unfav. Risk Cytogen. | 216 | 0.96 (0.65-1.43) |
| No AHD | 604 | 1.46 (1.13-1.89) |
| AHD | 123 | 1.22 (0.74-2.00) |
| De novo AML | 627 | 1.52 (1.18-1.96) |
| Therapy-related AML | 100 | 0.94 (0.54-1.61) |
| . | N . | HR CLO/Standard (95% CI) . |
|---|---|---|
| *Weighted OS | 727 | 1.41 (1.12-1.78) |
| Unweighted OS | 727 | 1.23 (1.00-1.50) |
| Age 60-69 | 449 | 1.48 (1.10-1.99) |
| Age 70+ | 278 | 1.34 (0.93-1.93) |
| Intermed. Risk Cytogen. | 378 | 1.77 (1.27-2.47) |
| Unfav. Risk Cytogen. | 216 | 0.96 (0.65-1.43) |
| No AHD | 604 | 1.46 (1.13-1.89) |
| AHD | 123 | 1.22 (0.74-2.00) |
| De novo AML | 627 | 1.52 (1.18-1.96) |
| Therapy-related AML | 100 | 0.94 (0.54-1.61) |
Conclusions: Despite similar CR & IM, OS after single agent CLO is inferior to standard DA therapy for pts age ≥60 years with newly diagnosed AML who are fit for intensive therapy, and DA remains the standard of care. However no difference in OS was observed after CLO in some pre-specified high risk AML subgroups. R#2 & AlloHCT arms continue in E2906 for pts already enrolled. Embedded prospective minimal residual disease study at CR is being performed to identify pts at higher risk after CLO & DA.
Weighted Kaplan-Meier Curves for OS
Unfavorable Cytogenetics OS by Therapy
Off Label Use: Use of clofarabine in AML, and maintenance therapy with decitabine in AML. Claxton:Medimmune: Research Funding; BMS: Consultancy; Astellas: Research Funding; Cyclacel: Research Funding; Merck: Research Funding; Ambit: Research Funding. Levine:Loxo Oncology: Membership on an entity's Board of Directors or advisory committees; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees; Foundation Medicine: Consultancy. Altman:Seattle Genetics: Consultancy; BMS: Consultancy; Spectrum: Consultancy; Astellas: Consultancy; Ariad: Consultancy; Novartis: Consultancy. Al-Kali:Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal