Abstract
Background: Chronic blood transfusions impact the liver due to accumulation of iron, leading to tissue damage, collagen formation and portal fibrosis. Long-term chelation therapy has been shown to be associated with stability or improvement of liver fibrosis and necroinflammation in heavily iron-overloaded patients with β thalassemia, independently of reductions in liver iron concentration (LIC) (Deugnier et al Gastroenterology 2011;141:1202-1211). It was also shown that changes in alanine aminotransferase (ALT, an indicator of hepatocellular damage) mirrored changes in LIC, with significant decreases in ALT seen in LIC responders. Here, we evaluated the effect of long-term chelation therapy on hepatocyte iron score (HIS), and the correlation of HIS and liver iron ratio (LIR) with ALT, to determine if the location of iron within the liver impacts liver function.
Methods: Study design, inclusion and exclusion criteria for studies 107 and 108 have been described previously (Deugnier et al, 2011). β thalassemia patients who had liver biopsy assessment at start of deferasirox treatment and after at least 3 years, were included in this analysis. Iron deposits were assessed according to size, cellular and lobular locations in Rappaport's acinus leading to three different scores: HIS (range 0-12), sinusoidal iron score (SIS, range 0-4) and portal iron score (PIS, range 0-4). The LIR, used to assess the relative amount of hepatocytic to total liver iron, was calculated as HIS/(HIS + SIS + PIS) x 100%. LIC was determined by liver biopsy. Correlation between ALT and HIS, and ALT and LIR was assessed using Pearson correlation coefficients. Baseline (BL) in these analyses refers to start of deferasirox treatment. Patients who received deferoxamine during the first year are referred to as the crossover cohort. Assessments were performed according to LIC response (see Table).
Results: Of 671 β thalassemia patients enrolled, 470 received chelation therapy for at least 3 years. Of these patients, 219 had histological biopsy data at BL and after at least 3 years of treatment. For all patients (n=219), mean absolute change ± standard deviation (SD) in HIS from BL (16.7 ± 7.4) to end of study (EOS; 11.8 ± 7.8) was -5.0 ± 9.3 (95% confidence interval [CI]; -6.3, -3.8), with a mean relative change of -18.1% (95% CI; -27.7, -8.5). In the crossover cohort (n=94), the mean absolute change in HIS from BL (15.1 ± 7.1) to EOS (11.6 ± 8.0) was -3.8 ± 9.3 (95% CI; -5.8, -1.8), with a mean relative change of -10.4% (95% CI; -26.8, 6.0).
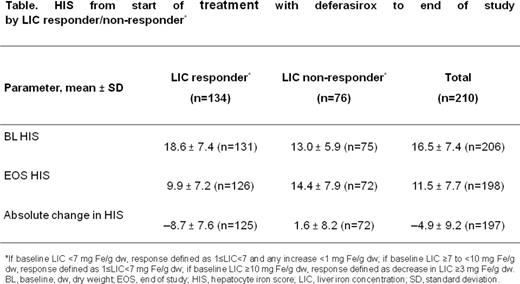
Among LIC responders, the mean absolute change of HIS from BL (18.6 ± 7.4) to EOS (9.9 ± 7.2) was -8.7 ± 7.6 (95% CI; -10.1, -7.4); mean relative change -43.5% (95% CI; -51.7, -35.4). Among LIC non-responders, the mean absolute change of HIS from BL (13.0 ± 5.9) to EOS (14.4 ± 7.9) was 1.6 ± 8.2 (95% CI; -0.3, 3.5); mean relative change 26.9% (95% CI; 6.9, 47.0; Table).
Correlation of HIS versus ALT: At BL and EOS, HIS showed a weak positive correlation with ALT in LIC responders (n=125, R=0.27 and n=126, R=0.31, respectively) and moderate correlation in non-responders (n=72, R=0.36 and n=72, R=0.43). Similarly, absolute change in HIS showed a weak correlation with change in ALT in LIC responders (n=125; R=0.17) with a moderate correlation in non-responders (n=72; R=0.44; Figure).
Correlation of LIR versus ALT: At BL and EOS, LIR showed no correlation with ALT in LIC responders (n=121; R=-0.07 and n=122; R=0.04, respectively) and non-responders (n=70; R=-0.14 and n=70; R=-0.05, respectively). Absolute change in LIR showed a weak negative correlation with change in ALT in LIC responders (n=121; R=-0.25) and no correlation in non-responders (n=70; R=0.02).
Discussion: Long-term iron chelation therapy is associated with improvement of liver function as measured by ALT in iron-overloaded patients with β thalassemia. Absolute change in HIS correlated with change in ALT in both LIC responders and non-responders. Although this correlation was weak to moderate, this finding supports the importance of excess iron removal from hepatocytes in order to improve ALT levels. By contrast, LIR which represents the proportion of HIS to total liver iron, showed no correlation. These results suggest that the improvement in liver function seen during chelation therapy may partly be due to the decrease in iron stored in hepatocytes. Further studies are warranted to investigate the mechanisms by which iron chelation therapy may improve liver function.
Cappellini:Novartis: Membership on an entity's Board of Directors or advisory committees; Genzyme/Sanofi: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Kattamis:ApoPharma: Speakers Bureau; Novartis: Research Funding, Speakers Bureau. Han:Novartis: Employment. El-Ali:Novartis: Employment. Porter:Celgene: Consultancy; Shire: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal