Abstract

Background:
Diseases whose treatment requires chronic transfusion therapy are relatively rare, and many have higher prevalence among certain ethnic groups and geographic regions. In these geographical regions, the patterns of care for these diseases and the epidemiology of iron overload (IOL) and other complications of treatment are currently undefined. To improve patient (pt) outcomes, it is important to understand how to diagnose, monitor, and manage these diseases. The TORS study aimed to collect information on a large number of newly diagnosed pts with various types of anemia and hemoglobinopathies to assess pt management considering diagnostic criteria and treatment pattern with iron chelation therapy (ICT) across various geographical regions.
Methods:
Inclusion and exclusion criteria were defined earlier by Siritanaratkul et al, EHA. 2015. Pts aged >2 years requiring chronic transfusion therapy with newly diagnosed anemias (<12 months from diagnosis), including low and intermediate-1 myelodysplastic syndromes (MDS), aplastic anemia (AA), and other transfusion-dependent (TD) anemias were enrolled. Pts were recruited from various geographical regions and were classified according to pt numbers, ethnicity and health care system as geo-1: Hong Kong, South Korea, Taiwan, Thailand; geo-2: China; geo-3: Tunisia, Morocco, Algeria, South Africa, Russia; geo- 4: Turkey. Pts were evaluated at baseline (BL) and at follow-up visits according to the standard practice for up to 3 years or until death.
Results:
Of the 564 pts (including 57 pts aged ≤18 years), 58.5% (n=330) were diagnosed with MDS, 31.2% (n=176) with AA, 10.1% (n=57) with other TD anemias. Diagnosis of 1 pt was missing.
The mean age (±SD) was 51.9±23.87 years (range, 2-92); 49.5% of pts (n=279) were male.
97.2% (321/330) of MDS pts were classified using the WHO classification. In 89.0% of MDS pts, a risk assessment according to IPSS score was performed.
Pts known to have received transfusions during the study include 92.6% (163/176) of AA, 68.4% (226/330) of MDS, and 93% (53/57) of other TD anemias. If analyzed by geographical region, 72.4% (126/174) of geo-1, 49.7% (99/119) of geo-2, 92% (115/125) of geo-3, and 70.5% (103/146) of geo-4 pts received transfusions during the study.
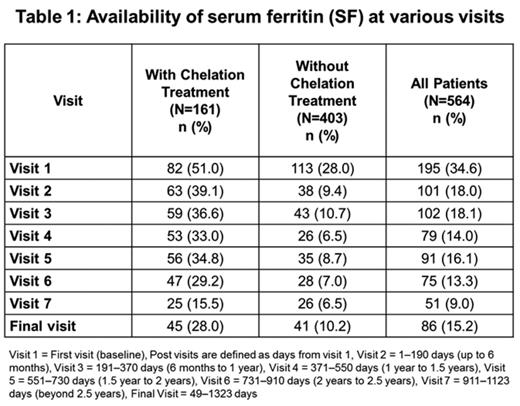
At BL, serum ferritin (SF) was only available in 34.5% (195/564) of overall pts (50.9% [82/161] of chelated; 28% [113/403] of non-chelated pts) (Table 1). If analyzed by disease, among chelated pts, 38.4% (15/39) of AA, 57.1% (52/91) of MDS, and 48.3% (15/31) of other TD anemias had BL SF values. SF was available in 35% (20/57) of pediatric pts at BL (39.2% [11/28] of chelated; 31% [9/29] of non-chelated pts).
In the overall pt population, data on ICT were available in 26% (13/50) of geo-1, 63.6% (7/11) of geo-2, 51.2% (20/39) of geo-3, and 68.8% (42/61) of geo-4 pts. At the final visit, the overall median change in SF from BL was -67.0 ng/mL.
Among the pts receiving ICT (n=161), median change in SF from BL was -325.6 ng/mL (-433.5 in AA, +483.5 in MDS, and -1113.0 in TD anemias) at final visit. Among the pts without ICT (n=403), the median change in SF from BL was +116.7 ng/mL (+116.2 in AA, +163.2 in MDS and -306.5 in TD anemias).
Among the pts with ICT (n=161), median change in SF from BL was +395 in geo-1, +113.5 in geo-3, and -433.5 in geo-4 at the final visit (pt data not available for geo-2). Among the pts without ICT (n=403), median change in SF from BL was +70.1 in geo-1, -170.6 in geo-2, -71.7 in geo-3, and +348.7 in geo-4 pts.
Conclusions:
In this large observational study, MDS was the most common disease type. This was potentially biased by site selection. Although the majority of pts received transfusion therapy leading to IOL, the awareness of IOL was low if measured by the availability of SF at BL and at each of the following visits. Consequently, many pts did not receive ICT. Interestingly, the majority of the MDS pts were classified according to WHO and stratified according to the recommended IPSS risk score, confirming that these guidelines are part of the standards of care in the clinical practice regardless of the geographic zone or the healthcare system. Overall, these results suggest that diagnosis and management practices of IOL and the underlying anemias may still be suboptimal in many parts of the world. Therefore, there is a need to improve local or regional understanding of IOL and its clinical consequences based on feasible therapeutic options in those regions.
Siritanaratkul:Janssen-Cilag: Research Funding; Novartis: Research Funding; Roche: Research Funding; Pfizer: Research Funding. Volodicheva:CELLTRION, Inc.: Research Funding. Wong:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Louw:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis Oncology: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. El-Ali:Novartis: Employment. Han:Novartis: Employment. Losco:Novartis: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal