Abstract

Background:
Autologous stem cell transplantation (ASCT) has been used as consolidation following induction therapy to reduce relapse (Le Gouill S et al et al, ASH 2014) in mantle cell lymphoma (MCL). Rituximab maintenance therapy post induction has been shown to improve overall survival (Kluin-Nelemans et al. NEJM 2012). Bortezomib has been shown to be effective against patients with relapsed/refractory MCL (Fisher et al, JCO 2006). However, the efficacy of the combination of bortezomib and rituximab as maintenance therapy post ASCT has not been established. Although CCND1 overexpression is pathognomonic in MCL, quantitative monitoring of CCND1 mRNA has not been established as a method of MRD monitoring. We aim to increase the 2 year disease free survival (DFS) for patients with MCL post ASCT with maintenance bortezomib and rituximab, and to explore the use of quantitative CCND1 mRNA expression as minimal residual disease (MRD) monitoring in this study. We report our interim analysis of DFS, toxicity, and quantitative CCND1 mRNA monitoring here.
Methods:
This is a multicenter phase II study in patients with MCL post ASCT. All patients were in complete remission (CT scan and bone marrow biopsy) post ASCT prior to entering study. Bortezomib was given at 1.3 mg/m2subcutaneously once a week for 4 weeks every 3 month for 2 years. Rituximabwas given at 375 mg/m2 intravenously once a week for 4 weeks every 6 months for 2 years. CT scanning and/or FDG-PETwere performed at every 6 months intervals to assess for disease status. The primary endpoint was 2 year DFS after start maintenance therapy. Secondary endpoints included toxicity, relapse rate, OS, and CCND1 mRNA as MRD monitoring. Blood was collected at baseline post ASCT and on day 1 of each cycle (3 month interval) at City of Hope. CCND1 mRNA was assessed using droplet digital PCR technology (ddPCR) on RNA obtained from peripheral blood mononuclear cells. JVM2 (MCL cell line), normal healthy patient, and an untreated patient with MCL involvement served as controls. All values were normalized to HPRT1 (housekeeping gene) and then to level of JVM2 (100%).
Results:
Sixteen patients were accrued, with 15 eligible for analysis. See Table 1 for baseline characteristics. The median follow- up was 19.1 months from time of starting maintenance therapy to time of last contact. The 2 year DFS is 100% (95% CI: N/A) and 2 year OS is 100% (95% CI: N/A). There have been no relapses and no treatment- related deaths on the study. Treatment was well tolerated. Grade 3/4 toxicities possiblyattributable to study drug included neutropenia (n=4), lymphopenia (3), pneumonia (1), and skin infection (1). Grade 2 possibly related toxicities included neutropenia (1), thrombocytopenia (2), anemia (1), arthralgia (1), peripheral sensory neuropathy (1), pruritis (1), and hypertension (1). Peripheral neuropathy toxicities included grade 1 sensory neuropathy (4), grade 1 motor neuropathy (1), and grade 2 sensory neuropathy (1).
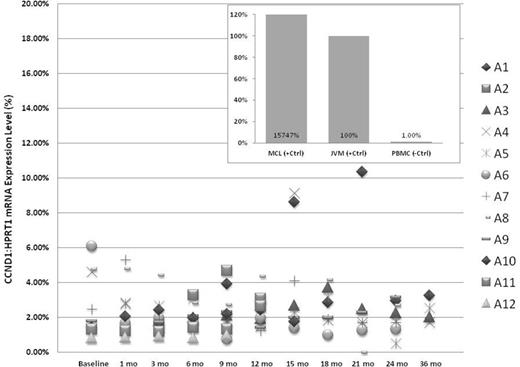
For MRD analysis, 12/15 patients had up to 12 months time point and 8/12 patients had up to 24 months time point. Out of 105 samples collected, ddPCR yield quantifiable CCND1 mRNA results in all time points tested except 3 samples. High CCND1 mRNA was detected in untreated patient with MCL involvement and JVM2. Low CCND1 mRNA was detected in normal healthy control and all samples on maintenance therapy. In all 12 patients analyzed, the relative CCND1 mRNA level was never above 12% of control (JVM2), and majority of the samples were below 5% (Figure 1). There were also very little intra-patient fluctuations.
Conclusions: The combination of bortezomib with rituximab for treating MCL is well tolerated post ASCT and has encouraging results with no relapses seen to date. Quantitative CCND1 mRNA monitoring by ddPCR is feasible and correlates with clinical outcome.
| Characteristics . | N (%) or Median (range) . |
|---|---|
| Median age | 62 (45-66) |
| Gender Male Female | 14 1 |
| Stage II Stage III Stage IV | 1 4 10 |
| Months from Transplant to Initial Maintenance | 3.1 (2.3-5.8) |
| Extra Nodal Disease No Yes | 4 11 |
| MIPI (At initial diagnosis) Low INT High | 8 6 1 |
| ASCT in 1st CR(n=13) -R-bendamustine -R-HCVAD/MTX/ARA-C -NORDIC -RCHOP ASCT in 2nd CR (n=2 ) -RCHOP followed by R-HCVAD/MTX/ARA-C -RCHOP followed by R-bendamustine | 3 4 4 2 1 1 |
| Median number of cycles | 6 (2-8) |
| Characteristics . | N (%) or Median (range) . |
|---|---|
| Median age | 62 (45-66) |
| Gender Male Female | 14 1 |
| Stage II Stage III Stage IV | 1 4 10 |
| Months from Transplant to Initial Maintenance | 3.1 (2.3-5.8) |
| Extra Nodal Disease No Yes | 4 11 |
| MIPI (At initial diagnosis) Low INT High | 8 6 1 |
| ASCT in 1st CR(n=13) -R-bendamustine -R-HCVAD/MTX/ARA-C -NORDIC -RCHOP ASCT in 2nd CR (n=2 ) -RCHOP followed by R-HCVAD/MTX/ARA-C -RCHOP followed by R-bendamustine | 3 4 4 2 1 1 |
| Median number of cycles | 6 (2-8) |
Quantitative CCND1 mRNA monitoring by ddPCR
Chen:Seattle Genetics, Inc.: Consultancy, Other: Travel expenses, Research Funding, Speakers Bureau; Genentech: Consultancy, Speakers Bureau; Millennium: Consultancy, Research Funding, Speakers Bureau. Off Label Use: 90-Y ibritumomab tiuxetan is a radiotherapeutic antibody targeting CD20. It is part of a regimen indicated for treating patients with relapsed/refractory, low-grade or follicular B cell non-Hodgkin lymphoma (NHL), or patients with previously untreated follicular NHL who achieved a complete or partial response to first-line chemotherapy.. Holmberg:Up to date: Patents & Royalties; Millennium: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genzyme: Membership on an entity's Board of Directors or advisory committees, Research Funding. Siddiqi:Seattle Genetics: Speakers Bureau; Kite pharma: Other: attended advisory board meeting; Pharmacyclics/Jannsen: Speakers Bureau. Nademanee:Gilead: Consultancy; Seattle Genetics Inc.: Research Funding; Spectrum: Research Funding; Celgene: Consultancy. Forman:Amgen: Consultancy; Mustang: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal