Abstract
Background: Proteasome-inhibitor (PI) based therapy is highly effective and widely utilized in the treatment of Waldenstrom's Macroglobulinemia (WM), though published data on the long-term impact of PI-based therapy, including treatment-related peripheral neuropathy and secondary malignancies remains limited.
Methods: We performed a prospective, multicenter study of bortezomib, dexamethasone and rituximab (BDR) in symptomatic, previously untreated WM patients. Treatment consisted of bortezomib 1.3 mg/m2 administered intravenously along with dexamethasone 40 mg on days 1, 4, 8, and 11; and rituximab 375 mg/m2 on day 11 as part of a 21-day cycle for 4 consecutive cycles as induction therapy. Maintenance therapy followed 12 weeks after induction therapy, and consisted of one cycle of BDR therapy every 12 weeks for a total of 4 cycles. Dose reduction or drug omission was permitted for toxicity. Herpes zoster prophylaxis and H2-blocker were required.
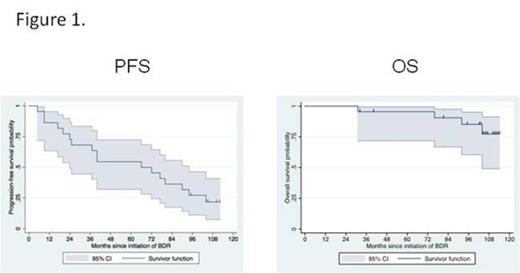
Results: Twenty-three patients received a median of seven cycles of treatment. The median baseline characteristics were as follows: age 66 years; bone marrow involvement 55%; serum IgM 4,830 mg/dL; serum IgA 45 mg/dL; serum IgG 418 mg/dL; hemoglobin 10.1 g/dL; and B2M 3.3 mg/L. Extramedullary disease was present in 4 (17%) patients. Following treatment, median serum IgM levels declined to 557 mg/dL (p<0.001), and median hemoglobin levels rose to 14.3 g/dL (p=0.001) at best response. At last assessment, the median serum IgA and IgG were 36 and 476 mg/dL (p=0.66 and 0.28, respectively versus baseline). Using updated consensus response criteria (Owen et al, BJH 2013), the overall and major response rates were 96% and 91%, respectively, and categorical responses were as follows: CR (N=4); VGPR (N=8); PR (N=9); MR (N=1). The median time to response was 1.4 months. With a median follow-up of 8.5 years, the median time to progression was 5.5 years, with an estimated 5-year progression free survival rate of 57% (95% CI 32-72%). Patients attaining a CR showed a longer progression-free survival interval (long rank p=0.03). The 5-year overall survival was 95% (95% CI 72-99%). Kaplan Meier curves for progression-free (PFS) and overall survival (OS) are shown in Figure 1. Four patients died during the follow-up period, with only one death related to WM (amyloid progression). Three patients had invasive malignancies that occurred at 29.5 (N=1; Breast CA); 44.7 (N=1; Prostate CA); and 83.8 (N=1; Vulvar CA) months following initiation of protocol therapy. No unexpected toxicities occurred. The most common grade >2 toxicities were as follows: peripheral neuropathy (N=16); neutropenia (N=13); infections without neutropenia (N=13); thrombocytopenia (N=10) and steroid related hyperglycemia (N=6). Discontinuance of bortezomib occurred in 14 (60%) patients for peripheral neuropathy; dexamethasone for steroid intolerance in 3 (13%) patients; and rituximab for antibody-related neutropenia in one patient (4%). For the 16 patients experiencing bortezomib related peripheral neuropathy, neuropathic complaints resolved (N=8); decreased to Grade 1 (N=5); remained unchanged (N=2); or were unevaluable (N=1) with prolonged follow-up.
Conclusions: BDR is a highly effective regimen producing high overall and major response rates, as well as long progression-free and overall survival intervals in previously untreated, symptomatic patients with WM. No unexpected toxicities were encountered. Secondary malignancies did not appear associated with protocol therapy. Reversible neurotoxicity constituted the most common adverse event associated with BDR using a twice-weekly schedule of administration of bortezomib, and resulted in high rates of proteasome-inhibitor discontinuance. Efforts to identify more neuropathy sparing approaches, including alternative schedules and routes of bortezomib administration, as well as novel neuropathy sparing proteasome-inhibitors are warranted given these encouraging long-term efficacy findings in WM.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal