Abstract
Introduction
Ibrutinib is a first-in-class, once-daily, oral, covalent inhibitor of BTK that has excellent single-agent activity in patients with previously treated CLL/SLL, based on results of published clinical studies. In the HELIOS trial, addition of ibrutinib to BR resulted in an 80% reduction in disease progression or death and confirmed for the first time, in a randomized setting, the benefit of ibrutinib-based therapy compared with standard chemoimmunotherapy (CIT) in previously treated patients (Chanan-Khan et al. ASCO 2015). In patients with CLL or SLL, deletion 17p (del17p) and 11q (del11q), complex karyotype, unmutated IgVH, and elevated ZAP70 are known high-risk prognostic factors. The current analysis reports on the efficacy of ibrutinib + BR vs placebo + BR in patients in the HELIOS trial with relevant high-risk biomarkers.
Methods
HELIOS is a randomized, double-blind, placebo-controlled, phase 3 study. Patients with active CLL/SLL following ≥ 1 prior therapy were randomized 1:1 to receive BR (≤ 6 cycles) with either ibrutinib (420 mg daily) (n = 289) or placebo (n = 289). Patients with del17p (> 20% of cells) were excluded. Ninety patients in the placebo arm with independent review committee (IRC)-confirmed progressive disease crossed over to ibrutinib, per protocol amendment. The primary end point was IRC-assessed progression-free survival (PFS), and overall survival (OS) was a secondary end point. A total of 287 patients in each arm had data available for at least 1 biomarker, and this population was used for the biomarker analyses. Correlation of biological risk factors at baseline with PFS and OS was analyzed using Kaplan-Meier analysis and curves compared using log-rank tests. Hazard ratios (HRs) were estimated using univariate Cox proportional hazards models.
Results
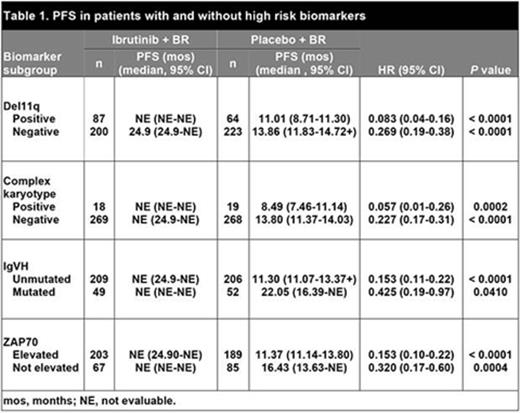
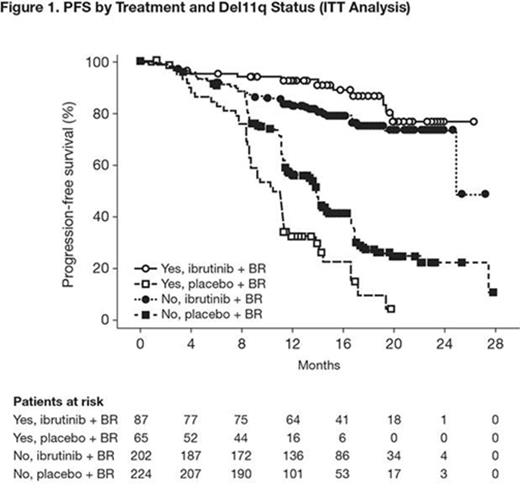
At a median follow-up of 17 months, IRC-assessed PFS was significantly longer with ibrutinib + BR vs placebo + BR in the overall study population (median not reached vs 13.3 months, respectively; HR: 0.203; 95% CI: 0.150-0.276; p < 0.0001). All subgroups of patients, including those with adverse prognostic parameters, e.g., del11q, complex karyotype, unmutated IgVH and elevated ZAP70 levels, had a significantly longer PFS with the addition of ibrutinib to BR as compared with placebo + BR (Table 1). As expected, each of these adverse prognostic parameters had a negative impact on PFS in the placebo + BR arm, e.g., the median PFS was shortest in patients with complex karyotype [8.5 months vs 13.8 months in patients without complex karyotype] and in patients with unmutated IgVH (11.3 months vs 22.1 months in patients with mutated IgVH). Interestingly, in the ibrutinib + BR arm, the adverse impact of these risk factors could not be detected (median PFS not reached irrespective of presence of risk factors). For example, in Figure 1 showing the PFS curves stratified by both del11q status and treatment, the outcome for patients in the ibrutinib + BR arm with del11q was similar to those without, with a trend toward better outcome in the del11q patients (e.g., longer PFS - not reached vs 24.9 months), unlike for patients in the placebo + BR arm (11.01 months vs 13.86 months). In patients with del11q, unmutated IgVH, or elevated ZAP70, there were also statistically significant benefits in OS for those receiving ibrutinib + BR vs placebo + BR (del11q: HR: 0.326, 95% confidence interval [CI], 0.122-0.870; p = 0.025; unmutated IgVH: HR: 0.515, 95% CI, 0.298-0.890: p = 0.017; elevated ZAP70, HR 0.536, 95% CI, 0.289-0.994: p = 0.048). Addition of ibrutinib also increased PFS in patient subgroups with other cytogenetic or clinical prognostic markers (such as del13q or bulky disease) compared with placebo.
Conclusions
All subgroups of patients, irrespective of adverse risk factors, such as del11q, complex karyotype, elevated ZAP70, and unmutated IgVH, benefit from the addition of ibrutinib to BR compared with placebo + BR. The negative impact of these known risk factors is apparent in patients in the placebo + BR arm, but is not seen in patients in the ibrutinib + BR arm. These results suggest that ibrutinib + BR is a suitable treatment regimen for all patients with previously treated CLL/SLL, including those with high-risk prognostic markers.
Cramer:Janssen: Other: Travel grant, Research Funding, Speakers Bureau; Hoffman LaRoche: Other: Travel grant, Research Funding, Speakers Bureau; Mundipharma: Other: Travel grant; Glaxo Smith Klein/Novartis: Research Funding; Astellas: Other: Travel grant; Gilead: Other: Travel grant, Research Funding. Fraser:Janssen: Honoraria, Research Funding, Speakers Bureau; Hoffman LaRoche: Consultancy, Honoraria; Celgene: Honoraria, Research Funding. Santucci Silva:Janssen: Other: Travel reimbursement, Research Funding; GSK: Research Funding; Celgene: Research Funding; Merck: Research Funding; Novartis: Other: Travel reimbursement; Hoffman LaRoche: Other: Travel reimbursement, Research Funding. Dilhuydy:Roche: Honoraria, Other: Travel reimbursement; Janssen: Honoraria, Other: Travel reimbursement; Mundipharma: Honoraria. Goy:Allos, Biogen Idec, Celgene, Genentech, and Millennium. Gilead: Speakers Bureau; Celgene: Consultancy, Research Funding, Speakers Bureau. Mato:Celgene: Consultancy, Other: Travel, Accommodations, Expenses, Research Funding; Gilead: Consultancy, Other: Travel, Accommodations, Expenses; Pharmacyclics LLC, an AbbVie Company: Consultancy, Other: Travel, Accommodations, Expenses; TA Therapeutics: Research Funding. Damle:Janssen: Employment. Phelps:Janssen/J&J: Employment, Equity Ownership. Mahler:Janssen: Employment, Other: Travel reimbursement. Salman:Janssen/J&J: Employment, Equity Ownership. Schaffer:Janssen: Employment. Howes:Janssen/J&J: Employment, Equity Ownership. Balasubramanian:Pharmacyclics LLC, an AbbVie Company: Equity Ownership; Janssen: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal